Amongst , the compounds with the greatest and the least ionic character, respectively, are.
Important Questions on Chemical Bonding and Molecular Structure
Arrange the following in increasing order of their covalent character.
(A)
(B)
(C)
(D)
Choose the correct answer from the options given below.
Which of the following statements are correct ?
(A) Both and are soluble in ethanol.
(B) The oxides and combine with excess of oxygen to give superoxide.
(C) is less soluble in water than other alkali metal fluorides.
(D) is more soluble in water than other alkali metal oxides.
Choose the most appropriate answer from the options given below
Order of Covalent bond;
A.
B.
C.
D.
E.
Arrange the following in the decreasing order of their covalent character :
(A)
(B)
(C)
(D)
Choose the most appropriate answer from the options given below
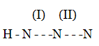
The above compound represents hydrogen azide, the bond orders of bonds and are:
Find out the correct order of ionic character in the following molecules

