An alkane is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of tertiary bromide. Write the structure of alkane and the tertiary bromide.

Important Questions on Hydrocarbons
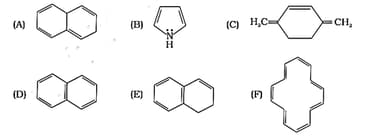
The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated bonds.
(ii) Complete delocalisation of the -electrons in ring system i.e. each atom in the ring has unhybridised p-orbital, and
(iii) Presence of -electrons in the ring where n is an integer [Huckel rule].
Using this information classify the following compounds as aromatic/ nonaromatic.
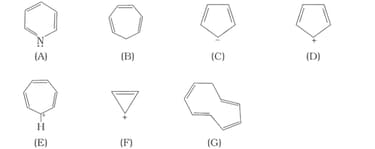
Which of the following compounds are aromatic according to Huckel’s rule?
Match the reagent from Column I which on reaction with gives some product given in Column II as per the codes given below:
| Column I | Column II | ||
| (i) | (a) | Acetic acid and | |
| (ii) | (b) | ||
| (iii) | (c) | ||
| (iv) | (d) | Acetaldehyde and formaldehyde | |
| (v) | (e) |
Match the hydrocarbons in Column I with the boiling points given in column II.
| Column I | Column II | ||
| (i) | n-Pentane | (a) | |
| (ii) | iso-Pentane | (b) | |
| (iii) | neo-Pentane | (c) |
Match the following reactants in Column I with the corresponding reaction products in Column II.
| Column I | Column II | ||
| (i) | Benzene | (a) | Benzoic acid |
| (ii) | Benzene | (b) | Methyl phenyl ketone |
| (iii) | Benzene | (c) | Toluene |
| (iv) | Toluene | (d) | Chlorobenzene |
| (e) | Benzene hexachloride |
