An aqueous solution of is electrolysed using direct current. The electrodes are inert and time dependence of current is represented as
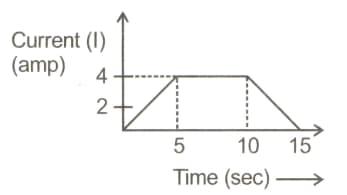
The mass (in ) of deposited at cathode is [Given ]


Important Points to Remember in Chapter -1 - Electrochemistry from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Electrochemical Cells:
For any electrode Oxidation potential Reduction potential.
Reduction potential of cathode Reduction potential of anode.
Reduction potential of cathode Oxidation potential of anode.
is always a positive quantity for a spontaneous reaction; Anode will be electrode of low reduction potential and Cathode will be of high reduction potential.
SRP of cathode SRP of anode. (SRP = Standard Reduction Potential)
The greater is the SRP value, the greater will be oxidising power.
2. Different types of Electrodes:
(i) Metal-metal ion electrode
(ii) Gas-ion Electrode
As reduction electrode
(iii) Oxidation-reduction electrode
As reduction electrode,
(iv) Metal-metal insoluble salt electrode
As reduction electrode, .
3. Gibbs Free Energy Change:
4. Nernst Equation:
(Effect of concentration and temperature on emf of cell)
(where is reaction quotient)
At chemical equilibrium: ;
5. Concentration Cell:
A cell in which both the electrodes are made up of the same material.
For all concentration cell,
(i) Electrolyte Concentration Cell:
(ii) Electrode Concentration Cell:
e.g.,
6. Calculation of different Thermodynamics Function of Cell Reaction:
(At constant pressure)
Temperature coefficient of emf of the cell.
of cell reaction:
7. Electrolysis:
(i)
(ii) Similarly, the anion which is stronger reducing agent (low value of ) is liberated first at the Anode.
8. Faraday's Law of Electrolysis:
(i) First Law:
Where Electrochemical equivalent of substance.
(ii) Second Law:
9. Conductance in Electrolytic Solutions:
(i)
(ii) Specific conductance or conductivity:
(Reciprocal of specific resistance) specific conductance
specific conductanceconductance
(iii) Equivalent conductance:
(iv) Molar conductance:
10. Kohlrausch's Law:
Variation of of a solution with concentration:
(i) Strong electrolyte
(ii) For both strong and weak electrolytes:
where is the molar conductivity.
No. of cations obtained after dissociation per formula unit.
No. of anions obtained after dissociation per formula unit.
11. Application of Kohlrausch’s Law:
(i) Calculation of of weak electrolytes:
(ii) To calculate degree of dissociation of a weak electrolyte
(iii) Solubilityof sparingly soluble salt & their
(iv) Ionic mobility: It is the distance travelled by the ion per second under the potential gradient of volts per . Its unit is .
(v) Absolute ionic mobility:
(vi) Transport Number:
Where Transport Number of cation & Transport Number of anions.
