EASY
NEET
IMPORTANT
Earn 100
An endothermic reaction is represented by the graph:
(a)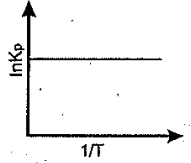

(b)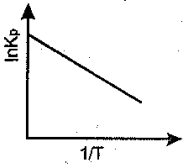

(c)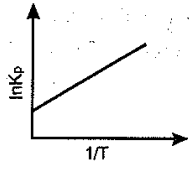

(d)None of these
100% studentsanswered this correctly

Important Questions on Equilibrium
HARD
NEET
IMPORTANT
, the correct set of thermodynamic parameters are
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Following species are classified into acid, base and amphiprotic species on the basis of protonic concept
()
()
()
()
()
()
()
which is correct match:
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
