EASY
NEET
IMPORTANT
Earn 100
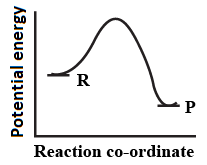
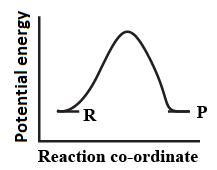
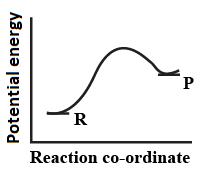
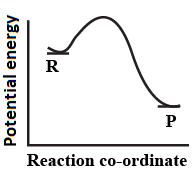
An endothermic reaction with high activation energy for the forward reaction is given by the diagram.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
The activation energy for a simple chemical reaction , is in the forward direction. The activation energy for the reverse reaction
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Activation energy of a chemical reaction can be determined by
EASY
NEET
IMPORTANT
The activation energy for a simple chemical reaction , is in the forward direction. The activation energy for the reverse reaction.
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Assertion: The rate law of a reaction cannot be predicted from its balanced chemical equation, but must be determined experimentally only.
Reason: The order of a reaction is always an integer like and .
[TS EAMCET (med.) 2015]
EASY
NEET
IMPORTANT
Which one of the following statement is not correct for the order of the reaction?
[IIT JEE 2005; CBSE PMT (Prelims) 2011]
