EASY
Earn 100
An engineer claims to have made an engine delivering power with fuel consumption of . The calorific value of the fuel is . The claim of the engineer
(a)is valid
(b)is invalid
(c)depends on engine design
(d)depends on the load
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
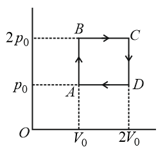
EASY
(Take cal = Joules)
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
HARD

