MEDIUM
NEET
IMPORTANT
Earn 100
An ideal gas expands in such a way that constant, throughout the process.
(a)The graph of the process of diagram is a parabola.
(b)The graph of the process of diagram is a straight line.
(c)Such an expansion is possible only with heating.
(d)Such an expansion is possible only with cooling
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
NEET
IMPORTANT
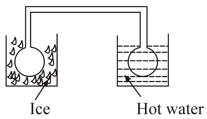
Two identical glass bulbs are interconnected by a thin glass tube at . A gas is filled at N.T.P. in this bulb is placed in ice and another bulb is placed in hot bath, then the pressure of the gas becomes times. The temperature of hot bath will be
MEDIUM
NEET
IMPORTANT
A gas has volume and pressure . The total translational kinetic energy of all the molecules of the gas is,
EASY
NEET
IMPORTANT
molecules of an ideal gas at temperature , and pressure are contained in a closed box. If the molecules in the box gets doubled, keeping total kinetic energy same. If new pressure is and temperature is , then
EASY
NEET
IMPORTANT
Assertion: If a gas container in motion is suddenly stopped, the temperature of gas rises.
Reason: The kinetic energy of ordered mechanical motion is converted into the kinetic energy of random motion of gas molecules.
EASY
NEET
IMPORTANT
Assertion: The root mean square and most probable speeds of the molecules in a gas are the same.
Reason: The Maxwell distribution for the speed of molecules in a gas is symmetrical.
