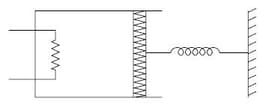
An ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section . Initially the gas is at 300K and occupies a volume of and the spring is in its relaxed state as shown in figure. The gas is heated by a small heater until the piston moves out slowly by 0.1 m. The force constant of the spring is 8000 N/m and the atmospheric pressure is The cylinder and the piston are thermally insulated. The piston and the spring are massless and there is no friction between the piston and the cylinder. The final temperature of the gas will be :
(Neglect the heat loss through the lead wires of the heater. The heat capacity of the heater coil is also negligible)
Important Questions on Thermodynamics
Consider the following thermodynamical variables
(i) Pressure
(ii) Internal Energy
(iii) Volume
(iv) Temperature
Out of these, the intensive variable(s) is (are)
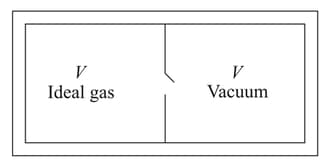
Consider the given diagram. An ideal gas is contained in a chamber (left) of volume and is at an absolute temperature It is allowed to rush freely into the right chamber of volume which is initially vacuum. The whole system is thermally isolated. What will be the final temperature, if the equilibrium has been attained?
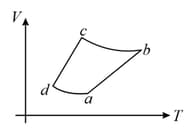
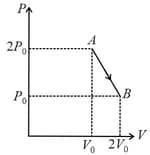
The corresponding P - V diagram for the process is (all figures are schematic and not drawn to scale) :
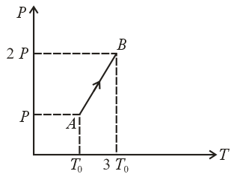
The pressure versus temperature graph of an ideal gas is shown in the figure below. If the density of gas at point is , then the density of the gas at point will be
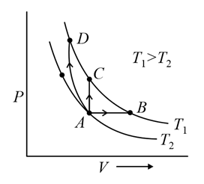
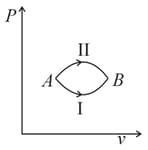
A system goes from to via two processes and shown in the figure. If and are the changes in internal energies in the processes I and Il respectively
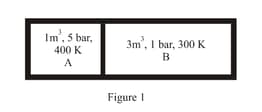
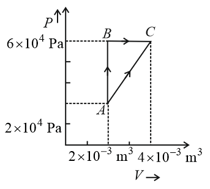
In process AB, of heat is added to the system and in process BC, of heat is added to the system. The heat absorbed by the system in the process AC will be:
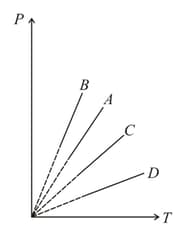
Pressure versus temperature graph of an ideal gas at constant volume is shown by the straight-line . Now mass of the gas is doubled and the volume is halved then the corresponding pressure versus temperature graph will be shown by the line
Three different processes that can occur in an ideal monoatomic gas are shown in the vs diagram. The paths are labelled as and . The change in internal energies during these process are taken as and and the work done as and . The correct relation between these parameters are: