MEDIUM
10th CBSE
IMPORTANT
Earn 100
An organic compound “A” of molecular formula on oxidation gives an acid “B” with the same number of carbon atoms in the molecule as “A”. Compound “A” is often used for sterilization of skin by doctors. Name the compounds “A” and “B”. Write the chemical equation involved in the formation of “B” from “A”.

Important Questions on Carbon and its Compounds
HARD
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
(a) ethanol to ethene.
(b) propanol to propanoic acid.
HARD
10th CBSE
IMPORTANT
What is the role of metal or reagents written on arrows in the given chemical reactions?
(a)
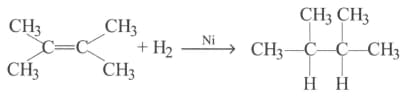
(b)
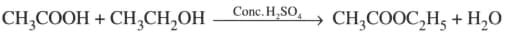
(c)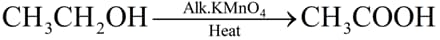
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
