Approximate values for the radius of a gold atom and the radius of a gold nucleus are and , respectively.
The density of gold is . Estimate the density of a gold nucleus, stating any assumptions that you make in your answer.

Important Questions on Atomic Structure and Particle Physics
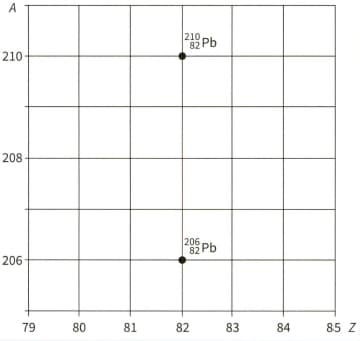
The nuclide of lead decays in three separate stages by and emission to another lead nuclide, .
-particles are known as ionising radiations. State and explain why such radiations can be described as ionising.
The nuclide of lead decays in three separate stages by and emission to another lead nuclide, .
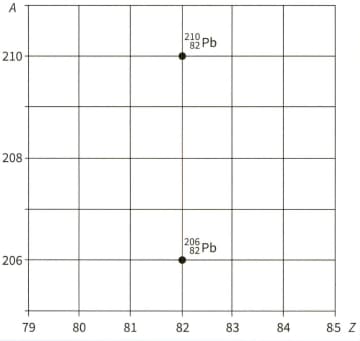
The two lead nuclides are shown in the graph, which plots nucleon number against proton number .
Copy the graph and, on your copy, draw three arrows to represent one possible route for the three decays between the two isotopes of lead. Label each arrow to show whether an -particle or -particle is emitted.
Geiger and Marsden carried out an experiment to investigate the structure of the atom. In this experiment, -particles were scattered by a thin film of gold.
When Rutherford analysed their results, what conclusions did he draw about the distribution of mass and charge in the atom?
Geiger and Marsden carried out an experiment to investigate the structure of the atom. In this experiment, -particles were scattered by a thin film of gold.
Describe and explain the experimental observations that led to these conclusions.
Beta decay occurs as either decay or - decay. An isotope of calcium Ca decays by emission into the isotope , and an isotope of magnesium decays by emission into the isotope .
Copy and complete the following decay equations for the calcium and magnesium isotopes.
Decay of calcium:
Beta decay occurs as either decay or - decay. An isotope of calcium Ca decays by emission into the isotope , and an isotope of magnesium decays by emission into the isotope .
(a) Copy and complete the following decay equations for the calcium and magnesium isotopes.
(ii) Decay of magnesium:
Beta decay occurs as either decay or - decay. An isotope of calcium Ca decays by emission into the isotope , and an isotope of magnesium decays by emission into the isotope .
State what happens in each type of -decay in terms of the quark model of nucleons.
decay
Beta decay occurs as either decay or decay. An isotope of calcium Ca decays by emission into the isotope , and an isotope of magnesium decays by emission into the isotope .
State what happens in decay in terms of the quark model of nucleons.
