HARD
Earn 100
Aqueous solution of two compounds and are prepared in two different beakers. If the electronegativity of and , then the nature of two solution will be respectively -
(a)Acidic, basic
(b)acidic, acidic
(c)basic, acidic
(d)basic, basic
50% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
Which of the following are Lewis acids?
EASY
The correct order of electronegativity for given elements is
EASY
In the following reactions, is respectively acting as a/an,
(i)
(ii)
HARD
In an acid-base titration, solution was added to the solution of unknown strength. Which of the following correctly shown the change of of the titration mixture in this experiment?
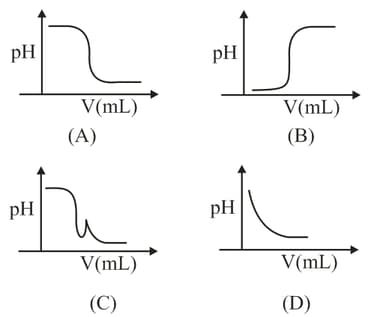
EASY
Which of the following indicates the correct variation in eletronegativities?
MEDIUM
Conjugate base for Bronsted acids and are:
MEDIUM
For standardizing solution, which of the following is used as a primary standard?
EASY
is an acid, according to
EASY
The most electronegative element from the following elements is:
EASY
Arrange as directed:
belonging to Group 16 of the long periodic table according to increasing order of electronegativity.
(The atomic numbers have been given within first brackets after the symbols of the elements)
MEDIUM
The strength of an aqueous solution is most accurately determined by titrating: (Note: consider that an appropriate indicator is used)
EASY
The electronegativity of the given elements increases in the order
EASY
In the reaction
functions as
MEDIUM
The electronegativities of and are in the order -
EASY
If you spill a chemical toilet cleaning liquid on your hand, your first aid would be:
EASY
The conjugate base of is
EASY
Which of the following is the strongest base?
EASY
Most electronegativity element in the periodic table
MEDIUM
Given that ionization potential and electron gain enthalpy of chlorine are and respectively. The electronegativity of chlorine on Mulliken scale, approximately equals to
EASY
Identify the correct order of electronegativity for and .

