Aqueous solution of which of the following compounds is the best conductor of electric current?
Important Questions on Equilibrium
(where the constant B is positive)
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
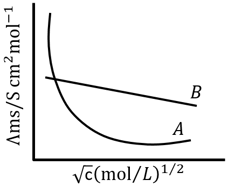
Following figure shows dependence of molar conductance of two electrolytes on concentration. is the limiting molar conductivity.
The number of Incorrect statement(s) from the following is _____
(A) for electrolyte is obtained by extrapolation
(B) For electrolyte graph is a straight line with intercept equal to
(C) At infinite dilution, the value of degree of dissociation approach zero for electrolyte .
(D) for any electrolyte or can be calculated using for individual ions.
Calculate the degree of dissociation of acetic acid, if its molar conductivity is .
Given: and .
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
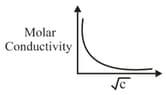
The electrolyte X is :
The electronic conductivity does not depend on