MEDIUM
11th CBSE
IMPORTANT
Earn 100
Arrange the elements in the order of increasing non-metallic character. Give the reason for the arrangement assigned.

Important Questions on Classification of Elements and Periodicity in Properties
HARD
11th CBSE
IMPORTANT
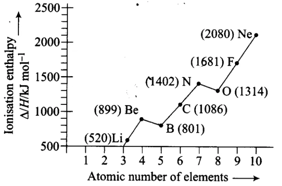
Explain the deviation In Ionisation enthalpy of some elements from the general trend by using Figure
MEDIUM
11th CBSE
IMPORTANT
Explain the following:
Electronegativity of elements increases on moving from left to right in the periodic table.
MEDIUM
11th CBSE
IMPORTANT
Explain the following:
Ionisation enthalpy decrease in a group from top to bottom?
MEDIUM
11th CBSE
IMPORTANT
How does the metallic and non-metallic character vary on moving from left to right in a period?
EASY
11th CBSE
IMPORTANT
The radius of cation is less than that of atom. Give reason.
EASY
11th CBSE
IMPORTANT
Among alkali metals which element do you expect to be least electronegative and why?
MEDIUM
11th CBSE
IMPORTANT
Match the correct atomic radius with the element.
| Element | Atomic radius (pm) | ||
| (i) | (a) | ||
| (ii) | (b) | ||
| (iii) | (c) | ||
| (iv) | (d) | ||
| (v) | (e) |
MEDIUM
11th CBSE
IMPORTANT
Match the correct ionisation enthalpies and electron gain enthalpies of the following elements.
| Elements | |||
| (i) | Most reactive nonmetal | (a) | |
| (ii) | Most reactive metal | (b) | |
| (iii) | Least reactive element | (c) | |
| (iv) | Metal forming binary halide | (d) |