HARD
Earn 100
Aryl halides are less reactive towards nucleophilic substitution reaction as compared to alkyl halide due to
(a)The formation of less stable carbonium ion
(b)Resonance stabilization
(c)longer carbon halogen bond
(d) sp2-hybridised carbon bonded to halogen
50% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
MEDIUM
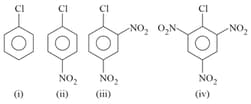
Arrange the following compounds in incrensing order of their rate of reaction towards hydroxyl ion .
I 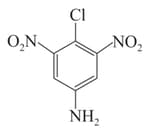
II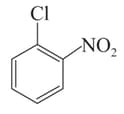
III 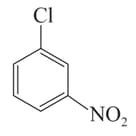
IV 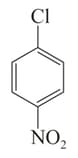
MEDIUM
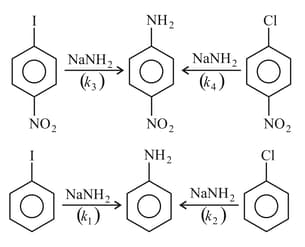
Identify the relation among the rates of following nucleophilic substitution reactions?
EASY
The correct order of the following compounds showing increasing tendency towards nucleophilic substitution reaction is:
EASY
MEDIUM
HARD
EASY
The rates of reaction of with:
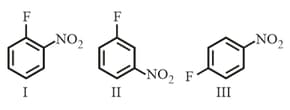
follow the order:-
EASY
MEDIUM
EASY
MEDIUM
Write the product formed when p-nitrochlorobenzene is heated with aqueous at 443 K followed by acidification.
MEDIUM
EASY
Give reasons:
The presence of the nitro group at 0/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
HARD
EASY
MEDIUM
How will you convert the following ?
- Chlorobenzene to Aniline
HARD
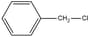 is a primary halide but it undergoes reaction as fast as tertiary halides. Give reason.
is a primary halide but it undergoes reaction as fast as tertiary halides. Give reason.EASY
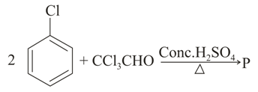
MEDIUM
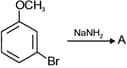
MEDIUM

