MEDIUM
Earn 100
Assertion: Phenol is more acidic than ethanol.
Reason: Phenoxide ion is resonance stabilized.
(a)Both assertion and reason are true and reason is the correct explanation of assertion
(b)Both assertion and reason are true but reason is not the correct explanation of assertion
(c)Assertion is true but reason is false
(d)Both assertion and reason are false
70% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
MEDIUM
MEDIUM
MEDIUM
Phenol to 2,4,6-tribromophenol
HARD
MEDIUM
MEDIUM
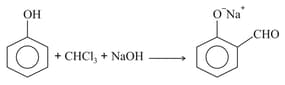
MEDIUM
Phenol to Salicylaldehyde
MEDIUM
The major product of the following reaction is:
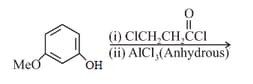
MEDIUM
MEDIUM
HARD
What is in the following sequence of reactions?
MEDIUM
| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
HARD
HARD
Phenol to Anisole
EASY
MEDIUM
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide
HARD
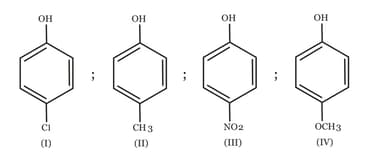
The increasing order of the values of the following compounds is:
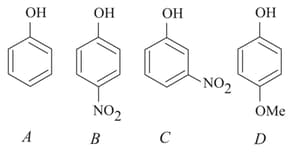
MEDIUM
Phenol to benzoquinone
MEDIUM
Account for the given statement :
nitrophenol is more steam volatile than -nitrophenol.
EASY
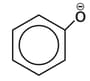 ion ?
ion ?
