EASY
NEET
IMPORTANT
Earn 100
At N.T.P. volume of a gas is changed to one fourth volume, at constant temperature then the new pressure will be :
(a)2 atm.
(b)25/3 atm.
(c)4 atm.
(d)1 atm.
100% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
NEET
IMPORTANT
A gas contained in a box of 0.1 m3 at atmospheric pressure is connected to another vessel of 0.09 m3. Consequent change in pressure is X mm of Hg. Then X in metre is -
MEDIUM
NEET
IMPORTANT
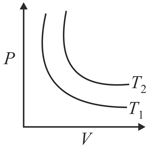
Find the correct relation in given P-V diagram:
EASY
NEET
IMPORTANT
Three particles have speeds of 2u , 10u and 11u. Which of the tollowing statements is correct?
EASY
NEET
IMPORTANT
The root mean square velocity of the molecules of an ideal gas is:-
EASY
NEET
IMPORTANT
At constant pressure hydrogen is having temperature of 327ºC. Till what temperature it is to be cooled so that the rms velocity of its molecules becomes half of the earlier value:-
EASY
NEET
IMPORTANT
The RMS velocity of gas molecules of a given amount of a gas at and pressure is . If temperature and pressure are, respectively, and , then the RMS velocity will be,
MEDIUM
NEET
IMPORTANT
Two container of same volume are filled with atomic Hydrogen and Helium respectively at 1 and 2 atm pressure. If the temperature of both specimen are same then average speed < CH > for hydrogen atoms will be-
EASY
NEET
IMPORTANT
The r.m.s. speed of a gas molecule is 300 m/s. Calculate the r.m.s. speed if the molecular weight is doubled while the temperature is halved-
