At moderate pressure, the compressibility factor of a particular gas is given by ( in bar and in Kelvin)
What is the Boyle's temperature of this gas?
Important Questions on States of Matter
A gas has a compressibility factor of and a molar volume of at a temperature of and pressure . If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be . The value of is _________
[Use: Gas constant, ]

Among the gases and , the gases that show only positive deviation from ideal behavior at all pressures in the graph are
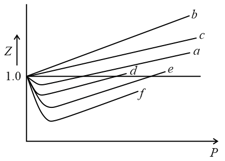
.
This equation reduces to the perfect gas equation, When,
The isotherms of a gas are shown below :
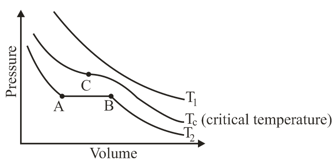
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
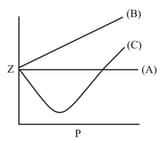
[Given : " a " and " b " are standard parameters for van der Waals' gas]

