HARD
JEE Main
IMPORTANT
Earn 100
At temperature above coke can be used to reduce to . How can you justify this reduction with the Ellingham diagram?

Important Questions on General Principles and Processes of Isolation of Elements
HARD
JEE Main
IMPORTANT
What chemical principle is involved in choosing a reducing agent for getting the metal from its oxide ore? Consider the metal oxides and and justify the choice of reducing agent in each case.
HARD
JEE Main
IMPORTANT
Although thermodynamically feasible, in practice, magnesium metal is not used for reduction of alumina in the metallurgy of aluminium. Why?
HARD
JEE Main
IMPORTANT
Free energy of formation of at and are given below:
at
at
at
at
At what temperature can be reduced by carbon?
HARD
JEE Main
IMPORTANT
In Ellingham diagram, the slope of the curve of the formation of metal oxide:
HARD
JEE Main
IMPORTANT
Which of the following statements is true?
EASY
JEE Main
IMPORTANT
Which of the following is correct regarding the given diagram of reduction of haematite?
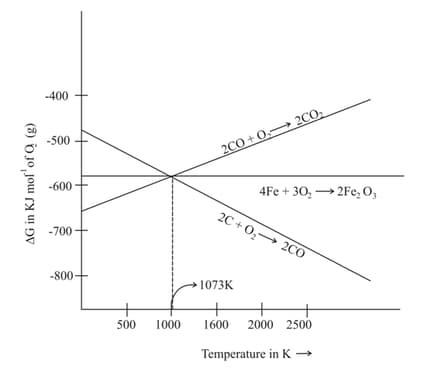
MEDIUM
JEE Main
IMPORTANT
vs plot in the Ellingham diagram slopes downward for the reaction:
MEDIUM
JEE Main
IMPORTANT
Consider the following reactions at
Choose the correct statement at .
