Atoms have no overall charge - they are neutral. Which one of the sentences below explains why?
They contain the same number of electrons and protons.
They contain the same number of protons and neutrons.
They contain neutrons, which have no charge.
The nucleus contains the same number of positive and negative particles.

Important Questions on The Periodic Table
The nucleus of an atom contains protons and neutrons. Which statement about the atom is true?
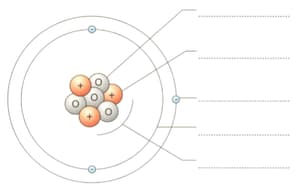
This is a diagram of a lithium atom. Label the diagram by choosing words from this list.
| period, group, proton, neutron, proton number, electron, nucleus, electron shell (orbit) |
The symbol for carbon in the Periodic Table is . Describe the electronic structure of a carbon atom.
These diagrams show two different models of the atom.
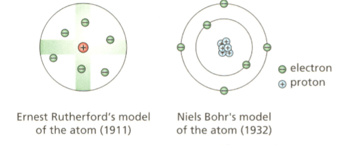
State two similarities and one difference between the two models.
Sodium has atomic number chlorine has atomic number . Compare the structure of a sodium atom with that of a chlorine atom.
Neon is in Group of the Periodic Table. Argon is below neon in the group. Which of these statements is true?
Read the following information about three Group elements.
At room temperature:
• bromine is a brown liquid
• iodine is a purple-black solid
• chlorine is a green gas.
Write down in order of increasing atomic number these three Group elements. Give a reason for your answer.
Magnesium and calcium are both in Group of the Periodic Table. Magnesium is above calcium. When magnesium is added to hydrochloric acid you can see bubbles of hydrogen gas. Predict what you will observe when calcium is added to some hydrochloric acid.
