HARD
Earn 100
Azide ion exhibits an bond order of 2 and may be represented by resonance structures I, II and III given below
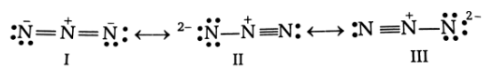
Select the correct statements.
(a)Structures I and II make greater contributions than III
(b)Structures II and III make greater contributions than I
(c)Structures I and III make greater contributions than II
(d)All three structures make equal contributions
30.77% studentsanswered this correctly
Important Questions on Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
HARD
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
In the given electron dot structure, the formal charge on each nitrogen atom (respectively) from left to right is _______
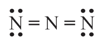
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.

