EASY
AS and A Level
IMPORTANT
Earn 100
Benzene can be nitrated to give nitrobenzene. Name the mechanism for this reaction.

Important Questions on Benzene and its Compounds
EASY
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. The species attacking benzene in the reaction is . How is generated in the reaction mixture? Name the substances used and give a chemical reaction leading to the formation of .
EASY
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. Give a suitable temperature for this reaction.
HARD
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. Use curly arrows to draw the mechanism of how benzene reacts with to produce nitrobenzene.
MEDIUM
AS and A Level
IMPORTANT
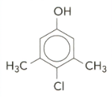
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
Give the molecular formula of the compound.
EASY
AS and A Level
IMPORTANT
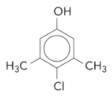
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
When bromine water is added to a solution of the compound, it is decolourised. Suggest two structures for the product of the reaction.
HARD
AS and A Level
IMPORTANT
Describe the bonding in benzene. Include a description of the model used for the arrangement of electrons in the molecules.
MEDIUM
AS and A Level
IMPORTANT
Cyclohexene decolourises bromine water whereas benzene has no effect on bromine water. Explain the difference in reactivity towards bromine water.