MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Benzene sulphonic acid is a stronger acid than benzoic acid, explain.

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main/Advance
IMPORTANT
Which is a stronger acid, or and why ?
HARD
JEE Main/Advance
IMPORTANT
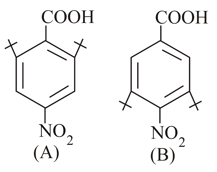
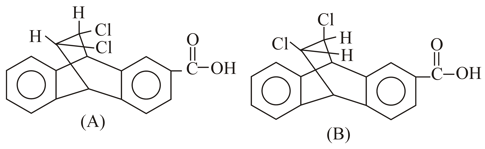
Which is a stronger acid, or and why ?
MEDIUM
JEE Main/Advance
IMPORTANT
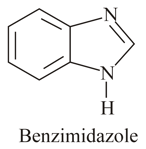
Discuss the basic strength of two nitrogens in benzimidazole.
EASY
JEE Main/Advance
IMPORTANT
In each of the following pair of compounds, which is more basic in aqueous solution ? Give an explaination for your choice :
(ii) or 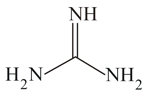
MEDIUM
JEE Main/Advance
IMPORTANT
Answer the following questions:
(i) Which of the indicated is abstracted rapidly by bromine radical and why?
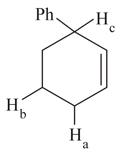
MEDIUM
JEE Main/Advance
IMPORTANT
Answer the following Questions:
(ii) One of the indicated proton or , is approximately times more acidic than other, which is more acidic and why?
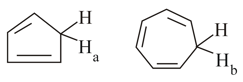
MEDIUM
JEE Main/Advance
IMPORTANT
Explain the following
(i) is a stronger acid than .
MEDIUM
JEE Main/Advance
IMPORTANT
Explain the following
(ii) is more acidic than .
