Brass is an alloy of copper and zinc. It is used to make brass musical instruments and to make electrical connectors and plugs. There are two main types of brass: and copper to zinc. The larger the amount ot zinc, the harder and stronger the alloy is.
Suggest which alloy is used for each of the purposes mentioned above. Give a reason for your answers.

Important Questions on Patterns and Properties of Metals
Caesium is an alkali metal. It is in Group I of the Periodic table.
State two physical properties of caesium.
Caesium is an alkali metal. It is in Group I of the Periodic table.
State the number of electrons in the outer shell of a caesium atom.
Complete the table below to estimate the boiling point and atomic radius of caesium. Comment also on the reactivity of potassium and caesium with water.
| Group I metal | Density | Radius of metal atom | Boiling point | Reactivity with water |
| Sodium | Floats and fizzes quickly on the surface, disappears gradually and does not burst into flame. | |||
| Potassium | _____ | |||
| Rubidium | Reacts instantaneously, fizzes and burst into flame then spits violently and may explode. | |||
| Caesium | _____ | _____ | _____ |
In each of the experiments below, a piece of metal is placed in a solution of a metal salt. Complete the table of observations.
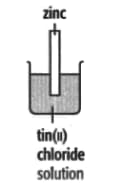 |
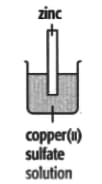 |
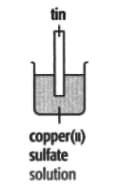 |
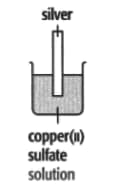 |
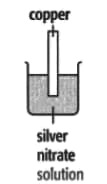 |
||
| At start | Colour of metal | grey | _____ | silver-coloured | silver-coloured | _____ |
| Colour of solution | colourless | _____ | blue | blue | colourless | |
| At finish | Colour of metal | coated with silver coloured crystals | _____ | coated with brown solid | silver-coloured | coated with silver-coloured crystals |
| Colour of solution | colourless | _____ | colourless | blue | _____ | |
