Bromine is a reddish-brown liquid. Some liquid bromine is placed in a closed jar. The bromine starts to evaporate. The colour of the vapour above the liquid bromine becomes darker and darker. After a time the bromine vapour does not get any darker. Use ideas about moving particles to explain these observations.

Important Questions on States of Matter
why is an alloy of copper and tin stronger than either copper or tin alone?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
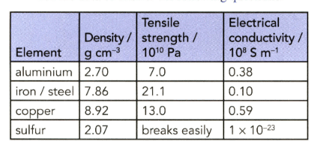
Why is aluminium with a steel core used for overhead electricity cables in preference to copper?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
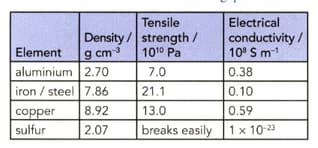
Suggest why many car engine blocks are made from aluminium alloys rather than from steel.
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
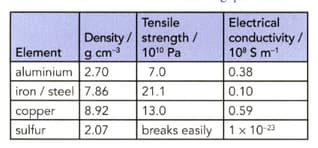
Explain the differences in tensile strength and electrical conductivity of iron and sulfur.
Explain the following property of silicon(IV) oxide by referring to its structure and bonding:
It has a high melting point.
It does not conduct electricity.
It is a crystalline solid.
