The reaction with 1-bromopentane proceeds by an mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.

Important Questions on Organic Chemistry [AHL]
Synthetic organic chemists often use a method referred to as retro-synthesis. Starting with knowledge of the structure and properties of the target compound, they think “in reverse” to determine possible synthetic pathways to produce it. Imagination, intuition, and reasoning all play their part in scientific innovation. Imagination transcends the limitations of acquired knowledge and opens up the possibility of new ideas.
What are the roles of these ways of thinking in solving synthetic pathway problems? Is retro synthesis a combination of understanding and imagination?
Which two molecules in given figure are cis–trans isomers of each other?
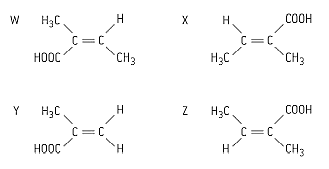
There are several structural isomers with the molecular formula .
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
The reaction with 2-bromo-2-methylbutane proceeds by an mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
Explain whether the boiling point of 1-bromopentane will be higher, lower, or the same as that of 2-bromo-2- methylbutane.
The product formed from the reaction with 1-bromopentane is warmed with ethanoic acid in the presence of a few drops of concentrated sulphuric acid. State the name of the type of reaction taking place and the structural formula of the organic product.
Deduce a multi-step synthesis for each of the following conversion. For each step state the structural formulae of the reactants and products and the conditions used for the reactions.
Propene to propyl ethanoate (2 steps)
