Calcium oxide reacts with hydrochloric acid according to the equation:
.
values:
Calculate the mass of water produced when calcium oxide reacts with excess of hydrochloric acid.

Important Questions on Atoms, Molecules and Stoichiometry
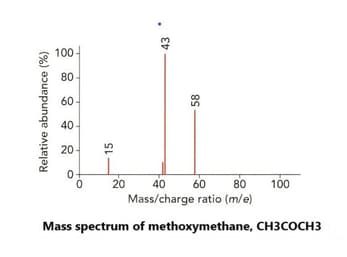
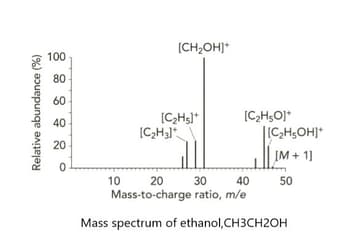
Look at the mass spectrum of ethanol, . A structural isomer of ethanol is methoxy methane, an ether with the formula .
Predict the mass-to-charge ratio of a fragment that would appear on the mass spectrum of methoxy methane but does not appear on methanol's mass spectrum.
Look at the mass spectrum of ethanol, . A structural isomer of ethanol is methoxy methane, an ether with the formula .
Predict and give the name of the fragment that would appear on the mass spectrum of methoxy methane but does not appear on methanol's mass spectrum.
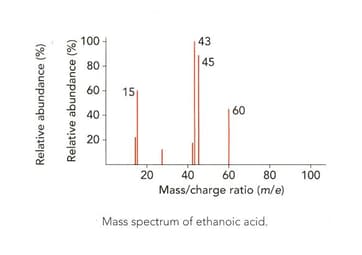
Look at the mass spectrum of ethanoic acid.
Identify the fragment with mass-to-charge ratio of .
A hydrocarbon has molecular ion peak at mass-to-charge ratio of (relative abundance ) and an peak with a relative abundance of .
How many carbon atoms are in the hydrocarbon?
When ammonia gas and hydrogen chloride gas mix together, they react to form a solid called ammonium chloride.
values:
Calculate the molar masses of ammonia, hydrogen chloride and ammonium chloride.
When ammonia gas and hydrogen chloride gas mix together, they react to form a solid called ammonium chloride.
values:
Calculate the volumes of ammonia and hydrogen chloride gases that must react at r.t.p in order to produce of ammonium chloride?
( of gas occupies at r.t.p.).