HARD
MYP:4-5
IMPORTANT
Earn 100
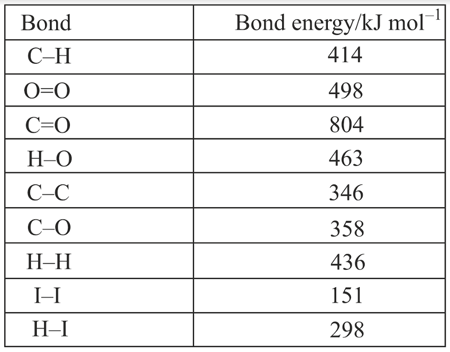
Calculate, using the information in the table, the enthalpy of combustion of ethanol.


Important Questions on How Can Our Energy Resources Be Accessed Fairly?
HARD
MYP:4-5
IMPORTANT
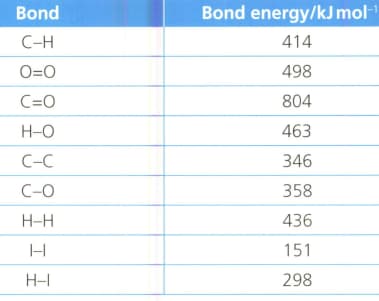
Calculate, using the information in the table, the enthalpy of combustion of octane , a major component of petrol.
HARD
MYP:4-5
IMPORTANT
Calculate the energy released when mole of methane is burnt.
MEDIUM
MYP:4-5
IMPORTANT
When of methane is burnt in oxygen, the heat evolved is . What is the heat of combustion (in ) of methane?
HARD
MYP:4-5
IMPORTANT
Calculate the energy released (heat produced) when of methane is burnt.
HARD
MYP:4-5
IMPORTANT
If a chemical reaction is positive: state the type of chemical reaction involved.
HARD
MYP:4-5
IMPORTANT
Describe the energy changes and the changes to the particle's movement and intermolecular forces when melting and boiling are reversed.
HARD
MYP:4-5
IMPORTANT
Identify the contribution of the components in Gibbs free energy when water boils and when it freezes.
MEDIUM
MYP:4-5
IMPORTANT
Explain why a burn from steam (at ) is more severe (painful) than a burn from boiling water (at ).
