Calculate the energy of a high-energy -photon, of frequency .

Important Questions on Quantum Physics
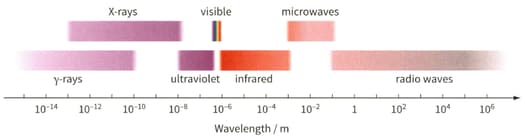
Determine the wavelength of the electromagnetic waves for the photon. Then use figure to identify the region of the electromagnetic spectrum to which it belongs.
The photon energy is:
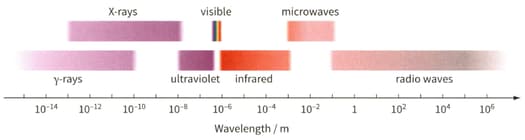
Determine the wavelength of the electromagnetic waves for the photon. Then use figure to identify the region of the electromagnetic spectrum to which it belongs.
The photon energy is:
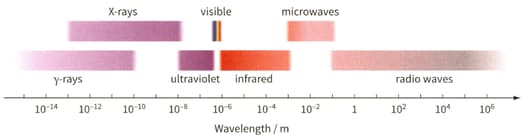
Determine the wavelength of the electromagnetic waves for the photon. Then use figure to identify the region of the electromagnetic spectrum to which it belongs.
The photon energy is:
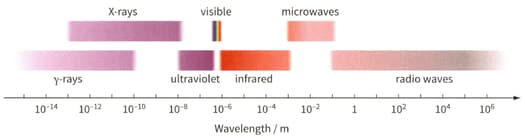
Determine the wavelength of the electromagnetic waves for the photon. Then use figure to identify the region of the electromagnetic spectrum to which it belongs.
The photon energy is:
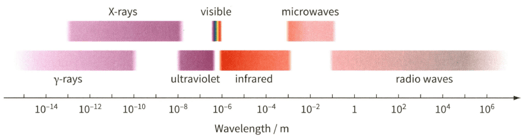
Determine the wavelength of the electromagnetic waves for the photon. Then use figure to identify the region of the electromagnetic spectrum to which it belongs.
The photon energy is:
A laser produces red light of wavelength . Calculate how many photons the laser produces per second.
