Calculate the volume occupied by 272 g of methane at a pressure of 250 kPa and a temperature of 54 °C.
(R = 8.31 JK–1mol–1; Mr methane = 16.0)

Important Questions on States of Matter
The pressure exerted by 0.25 mol of carbon monoxide in a 10 dm3 flask is 120 kPa. Calculate the temperature in the flask in Kelvin.
(R = 8.31 JK–1mol–1)
(R = 8.31 JK–1mol–1)
A flask of volume 2 x 10-3 m3 contains 4.19 g of a gas. The pressure in the flask is 300 kPa and the temperature is 20°C. R = 8.31 J K-1 mol -1.
You can find the relative molecular mass using the expression:
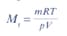
Which one of the following gives the correct value for the relative molecular mass of the gas?

why is an alloy of copper and tin stronger than either copper or tin alone?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
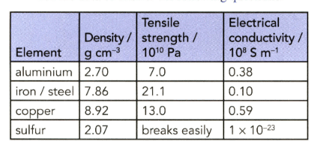
Why is aluminium with a steel core used for overhead electricity cables in preference to copper?
The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
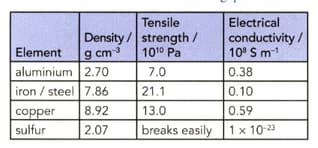
Suggest why many car engine blocks are made from aluminium alloys rather than from steel.
