HARD
Earn 100
Certain organic compound on combustion produces three gaseous oxides A, B and C. A and C turned lime water milky, B turned anhydrous blue and C turned solution green. The elements present in organic compounds are
(a)C, N, O
(b)C, H, S
(c)C, H only
(d)C, S only
60% studentsanswered this correctly
Important Questions on Some Basic Concepts of Chemistry
HARD
| Column – | Column - | ||
| A. | Aniline | i. | Red colour with |
| B. | Benzene sulfonic acid | ii. | Violet color with sodium nitroprusside |
| C. | Thiourea | iii. | Blue color with and acidic solution of |
EASY
| Item (Compound) | Item (Reagent) | ||
| Lysine | naphthol | ||
| Furfural | Ninhydrin | ||
| Benzyl alcohol | |||
| Styrene | Ceric ammonium nitrate | ||
EASY
EASY
MEDIUM
HARD
Match List-I with List-II
| List-I | List-II | ||
| Test/Reagents/Observation(s) | Species detected | ||
| (a) | Lassaigne's Test | (i) | Carbon |
| (b) | Cu(II) oxide | (ii) | Sulphur |
| (c) | Silver nitrate | (iii) | , and halogen |
| (d) | The sodium fusion extract gives black precipitate with acetic acid and lead acetate | (iv) | Halogen Specifically |
The correct match is :
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
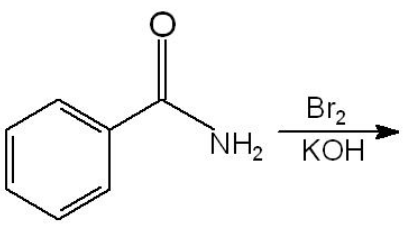
The major product is
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY

