EASY
Earn 100
Choose the polar molecule from the following :
(a)
(b)
(c)
(d)
80.77% studentsanswered this correctly
Important Questions on Chemical Bonding
MEDIUM
The bond lengths (in of and are:
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
How many ions of the following have a bond order of
EASY
MEDIUM
EASY
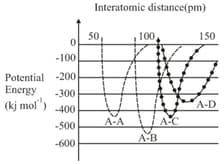
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
What will be the CORRECT order of the C-F bond distance among the following?
and
EASY
(A) and the other as Reason (R). Examine both the statements carefully and mark the correct choice.
(A) The bond angle in is greater than bond angle in
(R) Due to larger size of , hydrogen bonding does not occur in
MEDIUM
EASY
MEDIUM

