HARD
Earn 100
Compare the bond angles in NH3 and H3O+
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
HARD
EASY
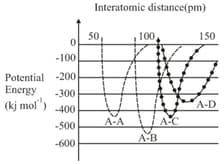
MEDIUM
EASY
How many ions of the following have a bond order of
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
The bond lengths (in of and are:
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM

