Compare the magnitude of hydration energy of alkaline earth metal ions in aqueous solution

Important Points to Remember in Chapter -1 - The s-Block Elements from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Electronic Configuration:
(i) All the alkali metals have one valence electron,
(ii) They readily lose an electron to give monovalent ions. Hence, they are never found in the free state in nature.
| Element | Symbol | Electronic configuration |
| Lithium | ||
| Sodium | ||
| Potassium | ||
| Rubidium | ||
| Caesium | or | |
| Francium |
2. Atomic and Ionic Radii:
(i) The alkali metal atoms have the largest sizes in a particular period of the periodic table.
(ii) The atomic and ionic radii of alkali metals increase on moving down the group i.e., they increase in size while going from to .
3. Ionization Enthalpy & Hydration Enthalpy :
(i) The ionization enthalpies of the alkali metals are considerably low and decrease down the group from to . This is because the effect of increasing size outweighs the increasing nuclear charge.
(ii) The hydration enthalpies of alkali metal ions decrease with increase in ionic sizes:
(iii) With increase in hydration, the size of ions in the solution increases and the ionic mobility and ionic conductance decreases.
4. Physical Properties of Group 1 Elements:
(i) All the alkali metals are silvery white, soft and light metals.
(ii) Because of the large size, these elements have low density which increases down the group from to . However, potassium is lighter than sodium.
(iii) The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them.
(iv) The alkali metals and their salts impart characteristic colour to an oxidizing flame. This is because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region as given below:
| Metal | |||||
| Colour | Crimson red | Yellow | Violet/Lilac | Red violet | Blue |
This property is used in characterization of alkali metals.
5. Chemical Properties of Group 1 Elements:
The alkali metals are highly reactive due to their larger size and low ionization enthalpy.
(i) Reactivity towards air: They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxide.
(a) Lithium forms monoxide, sodium forms peroxide, the other metals form superoxides. The superoxide ion is stable only in the presence of large cations such as , .
(b) The increasing stability of the peroxide or superoxide, as the size of the metal ion increases, is due to the stabilization of large anions by larger cations through lattice energy effects. These oxides are easily hydrolyzed by water to form the hydroxides according to the following reactions:
The oxides and the peroxides are colorless when pure, but the superoxides are yellow or orange in colour. The superoxides are also paramagnetic. Sodium peroxide is widely used as an oxidizing agent in inorganic chemistry.
(c) Lithium shows exceptional behaviour while reacting directly with nitrogen of air to form the nitride, as well.
(d) Because of their high reactivity towards air and water, alkali metals are normally kept in kerosene oil.
(ii) Reducing nature: The alkali metals are strong reducing agents, lithium being the most and sodium the least powerful.
(a) Solution in liquid ammonia: The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
The blue colour of the solution is due to the ammoniated electron and the solution is paramagnetic.
In concentrated solution, the blue colour changes to bronze colour and becomes diamagnetic.
(iii) Reactivity towards dihydrogen:
The alkali metals react with dihydrogen at about (lithium at ) to form hydrides. All the alkali metal hydrides are ionic solids with high melting points.
(iv) Reactivity towards halogens: The alkali metals readily react vigorously with halogens to form ionic halides, .
(v) Lithium halides are somewhat covalent. It is because of the high polarization capability of lithium ions (The distortion of electron clouds of the anion by the cation is called polarization). The ion is very small in size and has a high tendency to distort electron clouds around the negative halide ion. Since anion with large size can be easily distorted, among halides, lithium iodide is the most covalent in nature.
(vi) The alkali metal halides, , are all high melting, colourless crystalline solids.
(vii) The melting and boiling points always follow the trend: fluoride chloride bromide iodide.
(viii) All these halides are soluble in water. The low solubility of in water is due to its high lattice enthalpy whereas the low solubility of is due to smaller hydration enthalpy of its two ions. Other halides of lithium are soluble in ethanol, acetone and ethyl acetate; is soluble in pyridine also.
Reactivity towards water: The alkali metals react with water to form hydroxide and dihydrogen.
(ix) Lithium’s reaction with water is less vigorous than that of sodium. This behavior of lithium is attributed to its small size and very high hydration energy. Other metals of the group react explosively with water.
(x) The hydroxides which are obtained by the reaction of the oxides with water are all white crystalline solids.
(xi) The alkali metal hydroxides are the strongest of all bases and dissolve freely in water with evolution of much heat on account of intense hydration.
(xii) They also react with proton donors such as alcohol, gaseous ammonia and alkynes.
6. Uses of Group Elements:
(i) Lithium metal is used to make useful alloys, for example with lead to make ‘white metal’ bearings for motor engines, with aluminum to make aircraft parts, and with magnesium to make armor plates. It is used in thermonuclear reactions. Lithium is also used to make electrochemical cells.
(ii) Sodium is used to make a alloy needed to make and . These organolead compounds were earlier used as anti-knock additives to petrol, but nowadays vehicles use lead-free petrol. Liquid sodium metal is used as a coolant in fast breeder nuclear reactors.
(iii) Potassium has a vital role in biological systems. Potassium chloride is used as a fertilizer. Potassium hydroxide is used in the manufacture of soft soap. It is also used as an excellent absorbent of carbon dioxide.
(iv) Cesium is used in devising photoelectric cells.
7. Important Compounds of Group Elements:
The alkali metals form salts with all the oxo-acids. They are generally soluble in water and thermally stable. Their carbonates and in most cases the hydrogen carbonates also are highly stable to heat. As the electropositive character increases down the group, the stability of the carbonates and hydrogen carbonates increases. Lithium carbonate is not so stable to heat; lithium being very small in size polarises a large ion leading to the formation of more stable and . Its hydrogen carbonate does not exist as a solid.
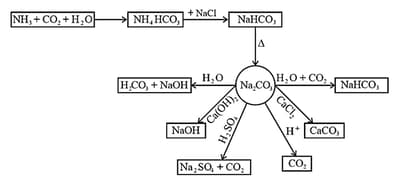
8. Sodium Carbonate (Washing Soda),
(i) Preparation:
(a) Sodium carbonate is generally prepared by Solvay Process. The equations for the complete process may be written as:
Sodium hydrogen carbonate crystal separates. These are heated to give sodium carbonate.
(b) is recovered when the solution containing is treated with Calcium chloride is obtained as a by-product.
(ii) Properties:
(a) It is readily soluble in water.
(b) On heating, the decahydrate loses its water of crystallization to form monohydrate. Above , the monohydrate becomes completely anhydrous and changes to a white powder called soda ash.
(c) Carbonate part of sodium carbonate gets hydrolyzed by water to form an alkaline solution.
(d) The other properties of sodium carbonate are given in the following chart
(iii) Uses:
(a) It is used in water softening, laundering and cleaning.
(b) It is used in the manufacture of glass, soap, borax and caustic soda.
(c) It is used in paper, paints and textile industries.
(d) It is an important laboratory reagent both in qualitative and quantitative analysis.
9. Sodium Chloride, .
(i) Occurrence:
The most abundant source of sodium chloride is sea water which contains to by mass of the salt. Common salt is generally obtained by evaporation of seawater. Approximately lakh tons of salt are produced annually in India by solar evaporation.
(ii) Purification:
(a) Crude sodium chloride, generally obtained by crystallization of brine solution, contains sodium sulphate, calcium sulphate, calcium chloride and magnesium chloride as impurity because they are deliquescent (absorb moisture easily from the atmosphere).
(b) To obtain pure sodium chloride, the crude salt is dissolved in a minimum amount of water and filtered to remove insoluble impurities. The solution is then saturated with hydrogen chloride gas. Crystals of pure sodium chloride separate out. Calcium and magnesium chloride, being more soluble than sodium chloride, remain in solution.
(iii) Properties:
(a) Sodium chloride melts at .
(b) It has a solubility of in of water at .
(c) The solubility does not increase appreciably with increase in temperature.
(iv) Uses:
(a) It is used as a common salt or table salt for domestic purposes.
(b) It is used for the preparation of and .
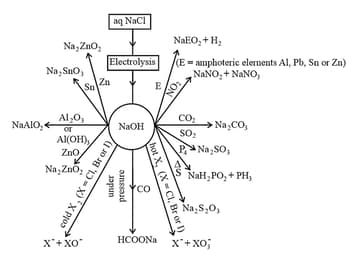
10. Sodium Hydroxide (Caustic Soda), .
(i) Preparation:
Sodium hydroxide is generally prepared commercially by the electrolysis of sodium chloride in Castner-Kellner cell: A brine solution is electrolysed using a mercury cathode and a carbon anode.
Cathode:
Anode:
The amalgam is treated with water to give sodium hydroxide and hydrogen gas.
(ii) Properties:
(a) Sodium hydroxide is a white, translucent solid.
(b) It melts at .
(c) It is readily soluble in water to give a strong alkaline solution.
(d) Crystals of sodium hydroxide are deliquescent.
(e) The sodium hydroxide solution at the surface reacts with the in the atmosphere to form .
(f) The other properties of sodium hydroxide given in the following chart
(iii) Uses:
(a) The manufacture of soap, paper, artificial silk, and a number of chemicals.
(b) In petroleum refining.
(c) In the purification of bauxite.
(d) In the textile industries for mercerizing cotton fabrics.
(e) For the preparation of pure fats and oils.
(f) As a laboratory reagent.
11. Sodium Hydrogen carbonate (Baking Soda),
(i) Preparation:
Sodium hydrogen carbonate is made by saturating a solution of sodium carbonate with carbon dioxide. The white crystalline powder of sodium hydrogen carbonate, being less soluble, gets separated out.
(ii) Uses:
(a) Sodium hydrogen carbonate is a mild antiseptic for skin infections.
(b) It is used in fire extinguishers.
12. Biological importance of sodium and potassium:
(i) A typical man contains about of and of compared with only of iron and of copper.
(ii) Sodium ions are found primarily on the outside of cells, being located in blood plasma and in the interstitial fluid which surrounds the cells. These ions participate in the transmission of nerve signals, in regulating the flow of water across cell membranes and in the transport of sugars and amino acids into cells.
(iii) Sodium and potassium, although so similar chemically, differ quantitatively in their ability to penetrate cell membranes, in their transport mechanisms and in their efficiency to activate enzymes. Thus, potassium ions are the most abundant cations within cell fluids, where they activate many enzymes, participate in the oxidation of glucose to produce and, with sodium, are responsible for the transmission of nerve signals.
(iv) Sodium-potassium pump operates across the cell membranes which consumes more than one-third of the used by a resting animal and about per in a resting human.
13. Anomalous Properties of Lithium:
The anomalous behaviour of lithium is due to the:
(i) exceptionally small size of its atom and ion.
(ii) high polarising power (i.e., charge/ radius ratio).
As a result, there is increased covalent character of lithium compounds which is responsible for their solubility in organic solvents.
Points of Difference between Lithium and other Alkali Metals:
(a) Lithium is much harder. Its m.p. and b.p. are higher than the other alkali metals.
(b) Lithium is least reactive but the strongest reducing agent among all the alkali metals. On combustion in air, it forms mainly monoxide, and the nitride, unlike other alkali metals.
(c) is deliquescent and crystallises as a hydrate, whereas other alkali metal chlorides do not form hydrates.
(d) Lithium hydrogen carbonate is not obtained in the solid form while all other elements form solid hydrogen carbonates.
(e) Lithium unlike other alkali metals forms no ethynide on reaction with ethyne.
(f) Lithium nitrate when heated gives lithium oxide, , whereas other alkali metal nitrates decompose to give the corresponding nitrite.
(g) and are comparatively much less soluble in water than the corresponding compounds of other alkali metals.
14. Points of Similarities between Lithium and Magnesium:
The similarity between lithium and magnesium is particularly striking and arises because of their similar sizes. The main points of similarity are:
(i) Both lithium and magnesium are harder and lighter than other elements in the respective groups.
(ii) Lithium and magnesium react slowly with water. Their oxides and hydroxides are much less soluble, and their hydroxides decompose on heating. Both form a nitride, and , by direct combination with nitrogen.
(iii) The oxides, and do not combine with excess oxygen to give any superoxide.
(iv) The carbonates of lithium and magnesium decompose easily on heating to form the oxides and . Solid hydrogen carbonates are not formed by lithium and magnesium.
(v) Both and are soluble in ethanol.
(vi) Both and are deliquescent and crystallise from aqueous solution as hydrates, and
15. General Introduction of Group Elements:
(i) Electronic Configuration:
Their general electronic configuration may be represented as . Like alkali metals, the compounds of these elements are also predominantly ionic.
| Element | Symbol | Electronic configuration |
| Beryllium | ||
| Magnesium | ||
| Calcium | ||
| Strontium | ||
| Barium | or | |
| Radium |
(ii) Atomic and Ionic Radii:
(a) The atomic and ionic radii of the alkaline earth metals are smaller than those of the corresponding alkali metals in the same periods. This is due to the increased nuclear charge in these elements.
(b) Within the group, the atomic and ionic radii increase with increase in atomic number.
(iii) Ionization Enthalpies:
(a) The alkaline earth metals have low ionization enthalpies due to the fairly large size of the atoms. Since the atomic size increases down the group, their ionization enthalpy decreases.
(b) The first ionization enthalpies of the alkaline earth metals are higher than those of the corresponding
Group metals. This is due to their small size as compared to the corresponding alkali metals.
(c) The second ionization enthalpies of the alkaline earth metals are smaller than those of the corresponding alkali metals.
(iv) Hydration Enthalpies:
(a) Like alkali metal ions, the hydration enthalpies of alkaline earth metal ions decrease with increase in ionic size down the group. .
(b) With increase in hydration, the size of ions in the solution increases and the ionic mobility and ionic conductance decreases.
(c) The hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions. Thus, compounds of alkaline earth metals are more extensively hydrated than those of alkali metals, e.g., and exist as and while and do not form such hydrates.
16. Physical Properties of Group Elements:
(i) The alkaline earth metals, in general, are silvery white, lustrous and relatively soft but harder than the alkali metals. Beryllium and magnesium appear to be somewhat greyish.
(ii) The melting and boiling points of these metals are higher than the corresponding alkali metals due to smaller sizes. The trend is, however, not systematic.
(iii) Because of the low ionization enthalpies, they are strongly electropositive in nature. The electropositive character increases down the group from to .
(iv) The alkaline earth metals like those of alkali metals have high electrical and thermal conductivities which are typical characteristics of metals
(v) Calcium, strontium and barium impart characteristic color to the flame.
| Metal | |||||
| Color | No Color | No Color |
Brick Red |
Crimson | Apple green |
17. Chemical Properties of Group Elements:
(i) Reactivity towards air and water:
(a) Beryllium and magnesium are kinetically inert to oxygen and water because of the formation of an oxide film on their surface. However, powdered beryllium burns brilliantly on ignition in air to give and . Magnesium is more electropositive and burns with dazzling brilliance in air to give and .
(b) Calcium, strontium and barium are readily attacked by air to form the oxide and nitride. They also react with water with increasing vigor even in cold to form hydroxides.
(c) All oxides except for , have rock-salt structure. The is essentially covalent in nature.
(d) is amphoteric while oxides of other elements are ionic in nature. All these oxides except are basic in nature and react with water to form sparingly soluble hydroxides.
(e) The solubility, thermal stability and the basic character of these hydroxides increase with increasing atomic number from to .
(f) The alkaline earth metal hydroxides are, however, less basic and less stable than alkali metal hydroxides.
(g) Beryllium hydroxide is amphoteric in nature as it reacts with acid and alkali both.
(ii) Reactivity towards the halogens:
(a) All the alkaline earth metals combine with halogen at elevated temperatures forming their halides.
(b) Thermal decomposition of is the best route for the preparation of , and is conveniently made from the oxide.
(c) Except for beryllium halides, all other halides of alkaline earth metals are ionic in nature.
(d) Beryllium halides are essentially covalent and soluble in organic solvents. Beryllium chloride has a chain structure in the solid state as shown below:
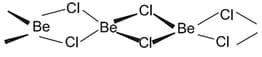
In the vapour phase tends to form a chloro-bridged dimer which dissociates into the linear monomer at high temperatures of the order of .
(e) The tendency to form halide hydrates gradually decreases (for example, , , and ) down the group. The dehydration of hydrated chlorides, bromides and iodides of and can be achieved on heating; however, the corresponding hydrated halides of and heating suffers hydrolysis.
(f) The fluorides are relatively less soluble than the chlorides owing to their high lattice energies.
(iii) Reactivity towards hydrogen:
(a) All the elements except beryllium combine with hydrogen upon heating to form their hydrides, .
(b) , however, can be prepared by the reaction:
(iv) Reactivity towards acids:
(a) The alkaline earth metals readily react with acids liberating dihydrogen.
(v) Reducing nature:
(a) The alkaline earth metals are a strong reducing agent. This is indicated by the large negative value of their reduction potentials.
(b) Solution in liquid ammonia: The alkaline earth metals dissolve in liquid ammonia to give deep blue-black solution forming ammoniated ions.
(c) From these solutions, the ammoniates, can be recovered.
18. Important Compounds of Group Elements:
(i) Carbonates:
(a) Carbonates of alkaline earth metals are insoluble in water and can be precipitated by addition of a sodium or ammonium carbonate solution to a solution of a soluble salt of these metals.
(b) The solubility of carbonates in water decreases as the atomic number of the metal ion increases.
(c) All the carbonates decompose on heating to give carbon dioxide and the oxide. The thermal stability increases with increasing cationic size.
(d) Beryllium carbonate is unstable and can be kept only in the atmosphere of .
(ii) Sulphates:
(a) The sulphates of the alkaline earth metals are all white solids and stable to heat.
(b) , and are readily soluble in water; the solubility decreases from to . The greater hydration enthalpies of and ions overcome the lattice enthalpy factor and therefore their sulphates are soluble in water.
(iii) Nitrates:
(a) The nitrates are made by dissolution of the carbonates in dilute nitric acid.
(b) Magnesium nitrate crystallizes with six molecules of water, whereas barium nitrate crystallizes as the anhydrous salt. This again shows a decreasing tendency to form hydrates with increasing size and decreasing hydration enthalpy.
(c) All of them decompose on heating to give the oxide like lithium nitrate.
(d) .
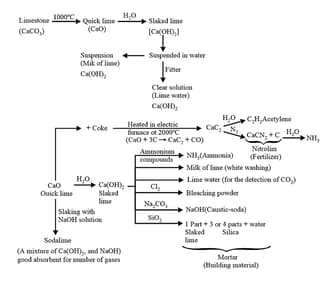
19.Calcium Oxide or Quicklime,
(i) Preparation:
It is prepared on a commercial scale by heating limestone in a rotary kiln at .
The carbon dioxide is removed as soon as it is produced to enable the reaction to proceed to completion.
(ii) Properties:
(a) Calcium oxide is a white amorphous solid.
(b) It has a melting point of .
(c) On exposure to the atmosphere, it absorbs moisture and carbon dioxide.
(d) The addition of a limited amount of water breaks the lump of lime. This process is called slaking of lime. Quick lime slaked with soda gives solid soda lime.
(e) Being a basic oxide, it combines with acidic oxides at high temperature.
(iii) Uses:
(a) It is an important primary material for manufacturing cement and is the cheapest form of alkali.
(b) It is used in the manufacture of sodium carbonate from caustic soda.
(c) It is employed in the purification of sugar and in the manufacture of dye stuff.
20. Calcium Hydroxide (Slaked lime),
(i) Preparation:
Calcium hydroxide is prepared by adding water to quick lime, .
(ii) Properties:
(a) It is a white amorphous powder.
(b) It is sparingly soluble in water.
(c) The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime.
(d) When carbon dioxide is passed through lime water it turns milky due to the formation of calcium carbonate.
On passing excess carbon dioxide, the precipitate dissolves to form calcium hydrogen carbonate.
(e) Milk of lime reacts with chlorine to form hypochlorite, a constituent of bleaching powder.
(iii) Uses:
(a) It is used in the preparation of mortar, a building material.
(b) It is used in whitewash due to its disinfectant nature.
(c) It is used in glass making, in the tanning industry, for the preparation of bleaching powder and for purification of sugar.
21. Calcium Carbonate, :
(i) Occurrence and preparation:
Calcium carbonate occurs in nature in several forms like limestone, chalk, marble etc. It can be prepared by passing carbon dioxide through slaked lime or by the addition of sodium carbonate to calcium chloride.
Excess of carbon dioxide should be avoided since this leads to the formation of water-soluble calcium hydrogen carbonate.
(ii) Properties:
(a) Calcium carbonate is a white fluffy powder.
(b) It is almost insoluble in water.
(c) When heated to , it decomposes to evolve carbon dioxide.
(d) It reacts with dilute acid to liberate carbon dioxide.
(iii) Uses:
(a) It is used as a building material in the form of marble and in the manufacture of quicklime.
(b) Calcium carbonate along with magnesium carbonate is used as a flux in the extraction of metals such as iron.
(c) Specially precipitated is extensively used in the manufacture of high-quality paper.
(d) It is also used as an antacid, mild abrasive in toothpaste, a constituent of chewing gum, and a filler in cosmetics.
22. Calcium Sulphate (Plaster of Paris), :
(i) Preparation:
It is a hemihydrate of calcium sulphate. It is obtained when gypsum, , is heated to .
Above , no water of crystallisation is left and anhydrous calcium sulphate, is formed. This is known as ‘dead burnt plaster’.
(ii) Properties:
It has a remarkable property of setting with water. On mixing with an adequate quantity of water it forms a plastic mass that gets into a hard solid in to minutes.
(iii) Uses:
(a) The largest use of Plaster of Paris is in the building industry as well as plasters.
(b) It is used for immobilizing the affected part of the organ where there is a bone fracture or sprain.
(c) It is also employed in dentistry, in ornamental work and for making casts of statues and busts.
(d) Other uses and properties are given in the following chart
23. Cement
(i) It is also called Portland cement because it resembles the natural limestone quarried in the Isle of Portland, England.
(ii) Cement is a product obtained by combining a material rich in lime, with other material such as clay which contains silica, along with the oxides of aluminum, iron and magnesium.
(iii) The average composition of Portland cement is: , ; , ; , ; , ; , and ,
(iv) For a good quality cement, the ratio of silica to alumina should be between and and the ratio of lime to the total of the oxides of silicon aluminum and iron should be as close as possible to .
(v) The important ingredients present in Portland cement are dicalcium silicate , tricalcium silicate and tricalcium aluminate .
(vi) Preparation:
The raw materials for the manufacture of cement are limestone and clay. When clay and lime are strongly heated together, they fuse and react to form a ‘cement clinker’. This clinker is mixed with by weight of gypsum to form cement.
(vii) Setting of cement:
(a) When mixed with water, the setting of cement takes place to give a hard mass. This is due to the hydration of the molecules of the constituents and their rearrangement.
(b) The purpose of adding gypsum is only to slow down the process of setting of the cement so that it gets sufficiently hardened.
(viii) Uses:
It is used in concrete and reinforced concrete, in plastering and in the construction of bridges, dams and buildings.
24. Biological importance of magnesium and calcium:
(i) An adult body contains about of and of
(ii) The daily requirement in the human body has been estimated to be .
(iii) All enzymes that utilize phosphate transfer requires magnesium as the cofactor. The main pigment for the absorption of light in plants is chlorophyll which contains magnesium.
(vi) About body calcium is present in bones and teeth. It also plays important roles in neuromuscular function, intraneuronal transmission, cell membrane integrity and blood coagulation. The calcium concentration in plasma is regulated at about . It is maintained by two hormones: calcitonin and parathyroid hormone.
25. Anomalous Properties of Beryllium:
(i) Beryllium has exceptionally small atomic and ionic sizes and thus does not compare well with other members of the group. Because of high ionization enthalpy and small size, it forms compounds which are largely covalent and get easily hydrolyzed.
(ii) Beryllium does not exhibit coordination number more than four as in its valence shell there are only four orbitals. The remaining members of the group can have a coordination number of six by making use of -orbitals.
(iii) The oxide and hydroxide of beryllium, unlike the hydroxides of other elements in the group, are amphoteric in nature.
26. Diagonal Relationship between Beryllium and Aluminum:
The ionic radius of is estimated to be ; the charge/radius ratio is nearly the same as that of the ion. Hence beryllium resembles aluminium in some ways. Some of the similarities are:
(i) Like aluminum, beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium hydroxide dissolves in excess of alkali to give a beryllate ion, just as aluminium hydroxide gives aluminate ion .
(iii) The chlorides of both beryllium and aluminum have bridged chloride structure in vapour phase. Both the chlorides are soluble in organic solvents and are strong Lewis acids. They are used as Friedel Craft catalysts.
(iv) Beryllium and aluminum ions have a strong tendency to form complexes, , .
27. Uses of Group 2 Elements:
(i) Beryllium is used in the manufacture of alloys. Copper-beryllium alloys are used in the preparation of high strength springs. Metallic beryllium is used for making windows of -ray tubes.
(ii) Magnesium forms alloys with aluminum, zinc, manganese and tin. Magnesium-aluminum alloys being light in mass are used in air-craft construction. Magnesium (powder and ribbon) is used in flash powders and bulbs, incendiary bombs and signals. A suspension of magnesium hydroxide in water (called milk of magnesia) is used as antacid in medicine. Magnesium carbonate is an ingredient of toothpaste.
(iii) Calcium is used in the extraction of metals from oxides which are difficult to reduce with carbon. Calcium and barium metals, owing to their reactivity with oxygen and nitrogen at elevated temperatures, have often been used to remove air from vacuum tubes.
(iv) Radium salts are used in radiotherapy, for example, in the treatment of cancer.
