HARD
Earn 100
Compare the physical properties of NaCl and methane.
Important Questions on Ionic and Covalent Bonding
EASY
MEDIUM
Answer the following:
does not conduct electricity in the solid state. Give reasons.
EASY
Give reasons.
(ii) Ionic compounds have high melting points.
EASY
HARD
MEDIUM
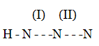
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
Why does distilled water not conduct electricity, whereas rain water does?
HARD
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM

