Compare these values of enthalpies of fusion and vaporization. Explain why the enthalpy of vaporization for a substance is greater than its enthalpy of fusion.
Enthalpy of fusion
Enthalpy of vaporization
Water
Carbon dioxide
Lead

Important Questions on Form
The boiling point of water is at an atmospheric pressure of atmosphere or , a typical level at sea level. At higher altitudes the air pressure decreases and the boiling point increases.
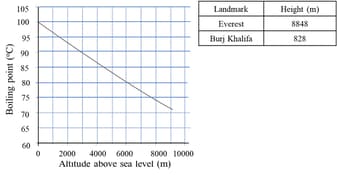
Study the graph and the table above. Use the information to calculate the percentage increase of the boiling point from the summit of Everest to the top of the Burj Khalifa.
The boiling point of water is at an atmospheric pressure of atmosphere or , a typical level at sea level. At higher altitudes the air pressure decreases and the boiling point increases.
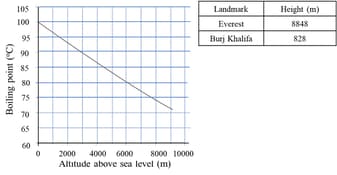
Will it take less time to cook pasta at the top Burj Khalifa or the summit of Everest? Explain your answer. What do you except to happen to the boiling point below sea level?
