Compound with molecular formula can show:

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
Choose the correct statement regarding the formation of carbocations A and B given :-
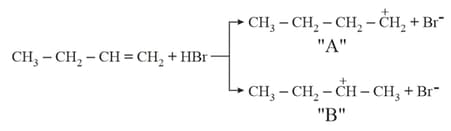
Given below are two statements:
Statement-I: Retardation factor can be measured in meter/centimetre.
Statement-II: value of a compound remains constant in all solvents.
Choose the most appropriate answer from the options given below:
Match List-I with List-II
| List-I | List-II | ||
| Test/Reagents/Observation(s) | Species detected | ||
| (a) | Lassaigne's Test | (i) | Carbon |
| (b) | Cu(II) oxide | (ii) | Sulphur |
| (c) | Silver nitrate | (iii) | , and halogen |
| (d) | The sodium fusion extract gives black precipitate with acetic acid and lead acetate | (iv) | Halogen Specifically |
The correct match is :
In Duma's method of estimation of nitrogen, of an organic compound gave of nitrogen collected at and of pressure. The percentage composition of nitrogen in the compound is ______. (Round off to the Nearest Integer).
[Given : Aqueous tension at of ]
Assertion A : Enol form of acetone exists in quantity. However, the enol form of acetyl acetone exists in approximately quantity.
Reason R : enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding, which is not possible in enol form of acetone.
Choose the correct statement:
