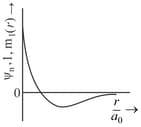
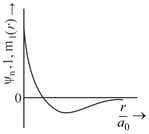
Consider a helium atom that absorbs a photon of wavelength . The change in the velocity (in ) of atom after the photon absorption is ______.
(Assume: Momentum is conserved when a photon is absorbed.
Use: Planck constant , Avocados number , Molar mass of )
Give your answer as the nearest integer.
(Assume: Momentum is conserved when a photon is absorbed.
Use: Planck constant , Avocados number , Molar mass of )

Important Questions on Structure of Atom
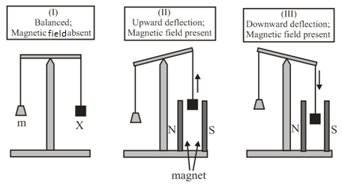
In an experiment, grams of a compound (gas/liquid/solid) taken in a container is loaded in a balance as shown in figure below. In the presence of a magnetic field, the pan with is either deflected upwards (figure ), or deflected downwards (figure ), depending on the compound . Identify the correct statement(s).
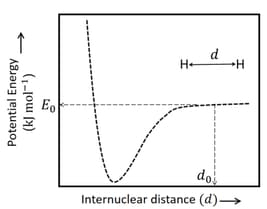
The figure below is the plot of potential energy versus internuclear distance of molecule in the electronic ground state. The value of the net potential energy (as indicated in the figure) is for at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent, find the value of to the nearest integer value.
As reference, the potential energy of atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as Give an answer to the nearest integer value.
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
(superoxide), dimeric sulphur in vapour phase,
| Column – 1 | Column – 2 | Column – 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite electron from state to state. |
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |