MEDIUM
Physics
IMPORTANT
Earn 100
Consider a thermodynamic cycle in a diagram shown in the figure performed on one mole of a monatomic gas. The temperature at is and volume at and are related as . Choose the correct option(s) from the following.
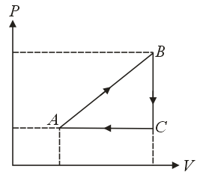

(a)The maximum temperature during the cycle is
(b)Net work done by the gas during the cycle is
(c)The heat capacity of the process is
(d)The efficiency of the cycle is
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
Physics
IMPORTANT
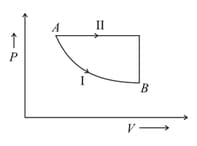
A certain mass of gas is taken from an initial thermodynamics state to another state by process I and II. In process I for the gas does of work and absorbs of heat energy. In process II, the gas absorbs of heat. The work done by the gas in process II is
MEDIUM
Physics
IMPORTANT
A vessel contains helium, which expands at constant pressure when of heat is supplied to it. The variation of the internal energy of the gas (in ) will be
MEDIUM
Physics
IMPORTANT
A vessel of volume contains hydrogen gas at temperature and pressure . The molar heat capacity of hydrogen at constant volume is . The heat (in ) required to raise the temperature to is
MEDIUM
Physics
IMPORTANT
If diagram of a diatomic gas is plotted, it is a straight line passing through the origin. The molar heat capacity of the gas in the process is where is an integer. The value of is
HARD
Physics
IMPORTANT
The value of is for an adiabatic process of an ideal gas for which internal energy . The value of ( is a constant) is
HARD
Physics
IMPORTANT
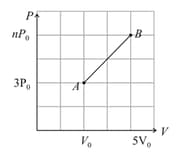
One mole of a monatomic ideal gas follows a process , as shown. The specific heat of the process is . The value of on -axis (see following figure) is
MEDIUM
Physics
IMPORTANT
Heat is supplied to of an ideal diatomic gas at temperature , which remains constant. Number of moles of the gas dissolved into atoms is
MEDIUM
Physics
IMPORTANT
A gaseous mixture enclosed in a vessel contains mole of a gas (with ) and another gas (with ) at a temperature . The gases and do not react with each other and are assumed to be ideal. The number of gram moles of if for the gaseous mixture is is:
