Consider one mole of a gas in a closed vessel fitted with a movable piston. At constant temperature , the pressure of the gas is increased gradually and the volume is noted. The resulting isotherm (not drawn to scale) is given below.
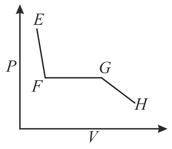
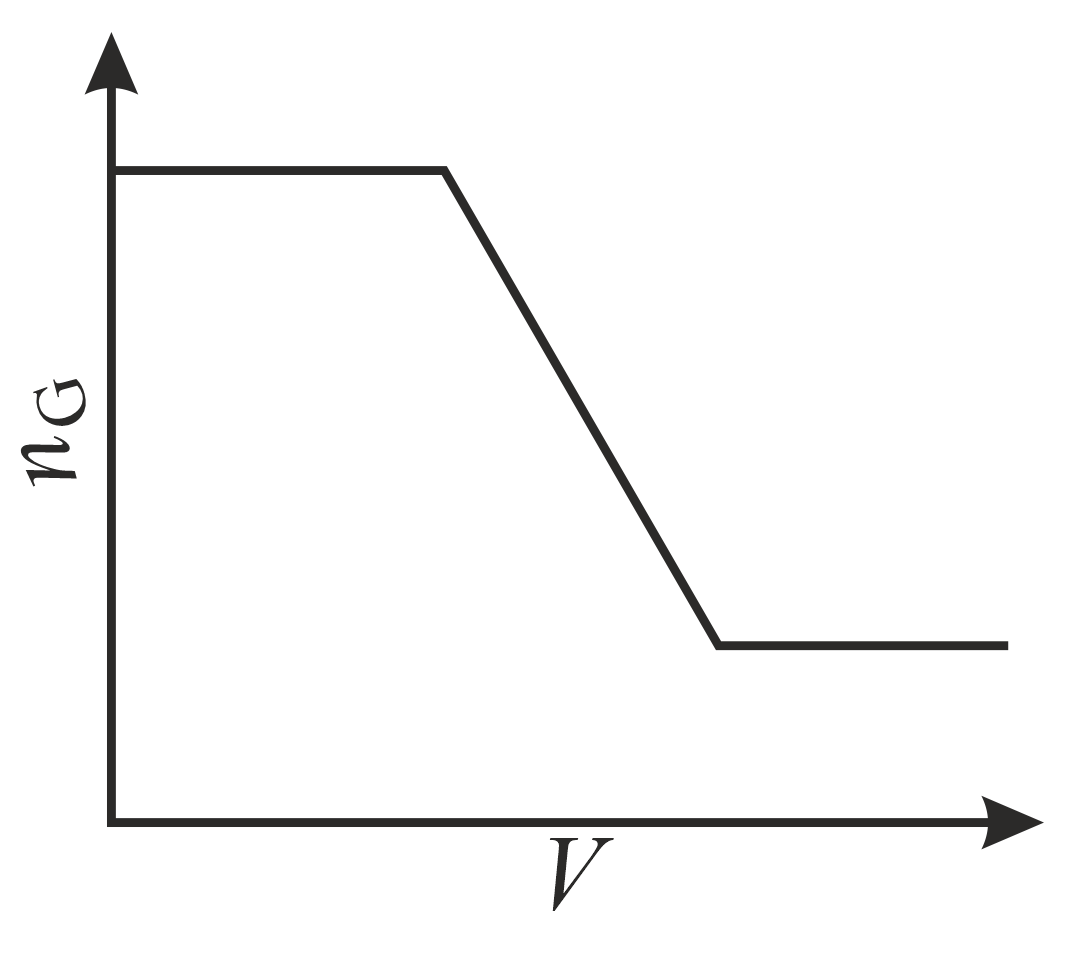
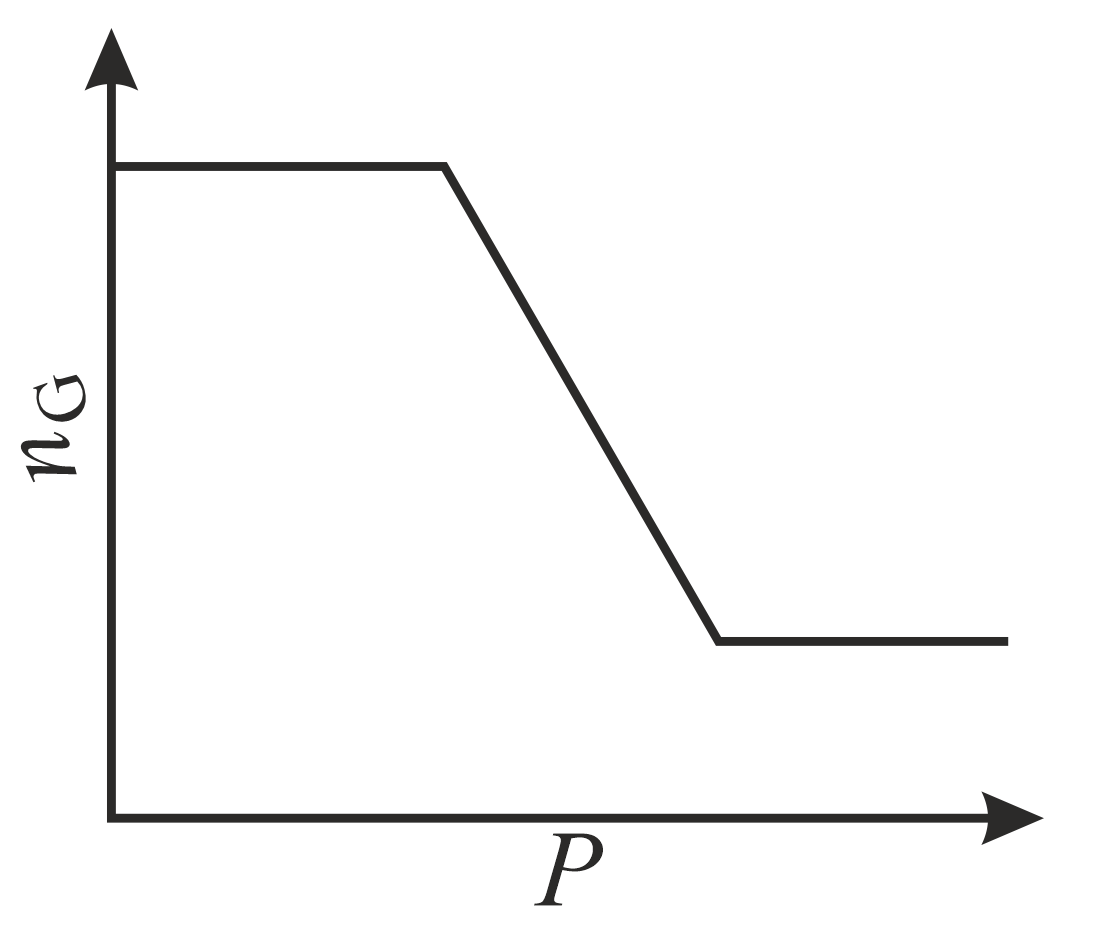
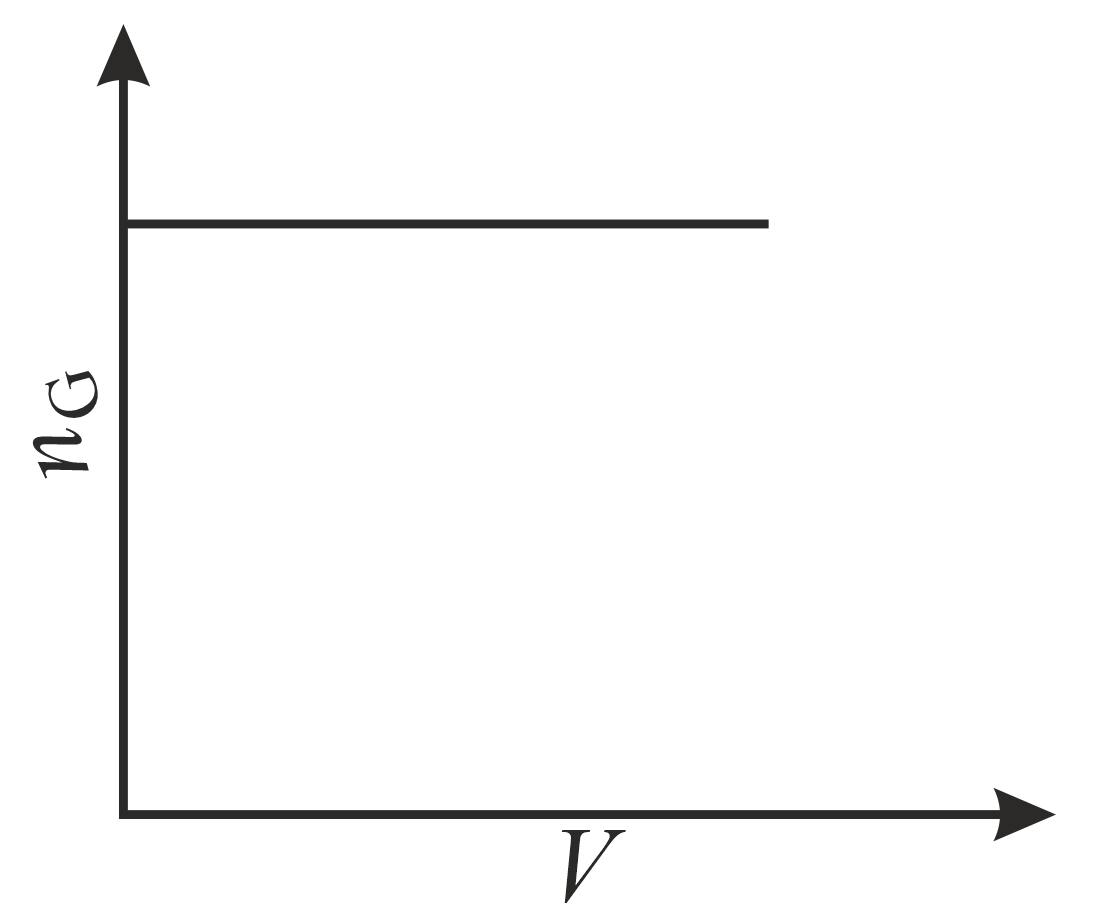
The correct plot (not drawn to scale) of the number of moles of the substance in gas phase $\left(n_{\mathrm{G}}\right)$ against $V$ or $P$ is:


Important Questions on States of Matter: Gases and Liquids
For the given isotherms, which of the following is correct for
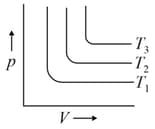
[Gas constant, ]
Choose the correct option for graphical representation of Boyle's law, which shows a graph of pressure vs. volume of a gas at different temperatures:
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
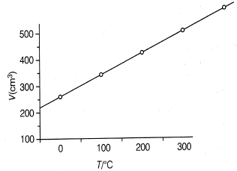
The volume of the gas at is larger than that at by a factor of

