EASY
NEET
IMPORTANT
Earn 100
Consider the following graph for the chemical reaction
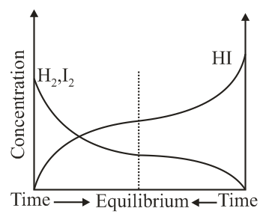
The inference that can be obtained from this graph about chemical equilibrium is that

(a)Equilibrium can be attained only when it starts from pure product
(b)Equilibrium can be attained only when it starts from pure reactants
(c)Equilibrium can be attained from both sides, either it starts from pure product or from pure reactants
(d)Equilibrium can't be attained in reverse direction if it starts from pure reactants
50% studentsanswered this correctly

Important Questions on Equilibrium
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
For the above two reactions which of the given relation is correct?
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
