HARD
Earn 100
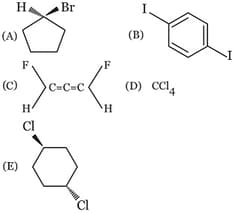
Consider the following haloalkanes
The correct sequence of increasing order of dipole moments is
(a)1 < 2 < 3 < 4
(b)4 < 3 < 2 < 1
(c)4 < 3 < 1 < 2
(d)3 < 4 < 1 < 2
79.1% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
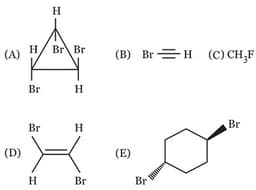
EASY
MEDIUM
MEDIUM
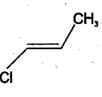 ?
?EASY
I. 1, 2, 3-trichloro benzene
II. 1, 4-dichloro benzene
III. 1, 2, 4-trichloro benzene
IV. 1, 3, 5-trichloro benzene

