Consider the following statement regarding Henry's law.
I. The solubility of solid in a liquid is directly proportional to temperature if it is endothermic reaction.
II. The solubility of a gas in a liquid is directly proportional to the partial pressure of gas present above the surface of liquid or solution.
III. The solubility of a liquid in a gas is directly proportional to the partial pressure of liquid present above the surface of gas.
IV. The solubility of a gas in solid is directly proportional to the partial pressure of gas present above the surface of solid.
Choose the correct statement (s).

Important Questions on Water
The solubility of salts increases with an increase in temperature. The graph given below shows the solubility of different salts at different temperatures.
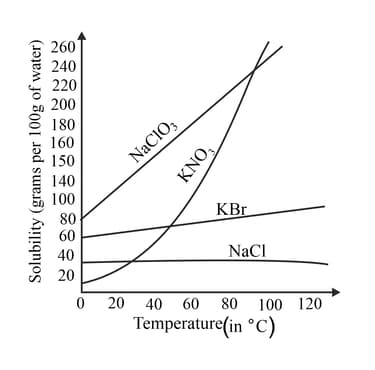
From the information given in the graph, which salt is almost equally soluble at all temperatures ?
Which one of the following graph shows solubility of a gas in a liquid?
A solution contains of common salt in of water. The concentration of the solution is
X is a hydrate of calcium. On heating, it gives an another hydrate, Y which is used for setting fractured bones. The ratio of water molecules in X and Y (X: Y) is
One mole of a sample of hydrated sodium sulphide contains of water of crystallisation. What is the correct formula of this compound?
A compound X of sodium is commonly used in kitchen to make crispy pakoras. It is also an active ingredient for curing acidity in the stomach. What is the product formed when solution of X is heated and the obtained product is recrystallised?
