Consider the graph for formation of H2 molecule from two H atoms
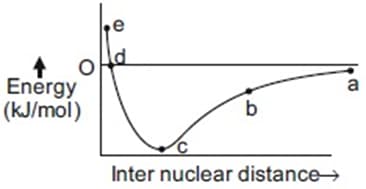
The point which corresponds to the formation of chemical bond is
Important Questions on Chemical Bonding and Molecular Structure
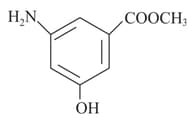
Given below are two statements.
Statement I: The presence of weaker -bonds make alkenes less stable than alkanes
Statement II: The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options : given below.
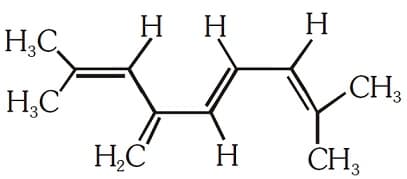
The total number of and bonds present in the following compound are
Given below are two statements : One is labelled as Assertion and the other is labelled as Reason
Assertion : Zero orbital overlap is an out of phase overlap.
Reason : It results due to different orientation/ direction of approach of orbitals.
In the light of the above statements. Choose the correct answer from the options given below
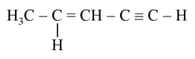 is _______.
is _______.The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
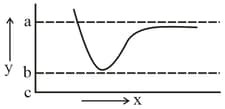
The bond energy of is :

