HARD
Earn 100
Convert into °C (Celsius scale).
Important Questions on Thermal Properties of Matter
EASY
The specific heat of water and the latent heat of ice . of ice at is placed in of water at . The amount of ice that will melt as the temperature of water reaches is close to (in grams)
MEDIUM
The specific heat capacity of a substance is and that of a substance is . Which of the two substances is a good conductor of heat? Give a reason for your answer.
EASY
A calorimeter of water equivalent contains of water at . ' grams of steam at is mixed in it till the temperature of the mixture is . The value of is close to (Latent heat of water, specific heat of water)
EASY
Two moles of oxygen is mixed with eight moles of helium. The effective specific heat of the mixture at constant volume is
HARD
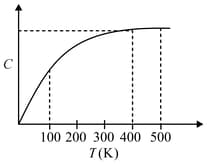
The figure below shows the variation of specific heat capacity () of a solid as a function of temperature (). The temperature is increased continuously from to at a constant rate. Ignoring any volume change, the following statement (s) is (are) correct to a reasonable approximation.
EASY
The specific heat capacity of body depends on
EASY
State whether the specific heat capacity of a substance remains the same when its state changes from solid to liquid? Give one example to support your answer.
MEDIUM
Why does stone lying in the sun get heated up much more than water lying for the same duration of time?
HARD
A bullet whose specific heat is, and moving at, plunges into a block of wax whose specific heat is, . Both bullet and wax are at, and assume that the bullet comes to rest in the wax and all its kinetic energy goes into heating the wax. The thermal temperature of the wax in, is close to.
HARD
of ice at is mixed with of water at . Assuming that there is no loss of energy to the environment, what will be the final temperature of the mixture? (Assume latent heat of ice , specific heat of water and ice are and , respectively.)
MEDIUM
Boiling water is changing into steam. The specific heat of boiling water is
EASY
water is heated from to . Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water ):
EASY
What is specific heat capacity?
MEDIUM
Steam at is passed into of water contained in a calorimoter of water equivalent to at till the temperature of the calorimeter and its contents rises to . The mass of steam condensed (in ) is
MEDIUM
Two metallic blocks P and Q having masses in ratio are supplied with the same amount of heat. If their temperatures rise by the same degree, compare their specific heat capacities.
EASY
Define heat capacity of a substance.
EASY
Write the SI unit of heat capacity.
MEDIUM
Water of volume 2 L in a closed container is heated with a coil of 1 kW. While water is heated, the container loses energy at a rate of 160 J/s. In how much time will the temperature of water rise from 27oC to 77oC ? (Specific heat of water is 4.2 kJ/kg and that of the container is negligible).
MEDIUM
An experiment takes to raise the temperature of water in a container from to and another to convert it totally into steam by a heater supplying heat at a uniform rate. Neglecting the specific heat of the container and taking specific heat of the water to be , the heat of vaporization according to this experiment will come out to be:
HARD
A water cooler of storage capacity litres can cool water at constant rate of . In a closed circulation system (as shown schematically in the figure), the water from the cooler is used to cool an external device that generates constantly of heat (thermal load). The temperature of water fed into the device can not exceed and the entire stored litres of water is initially cooled to . The entire system is thermally insulated. The minimum value of (in ) for which the device can be operated for hours is:
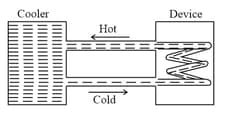
(Specific heat of water is and the density of water is )

