HARD
Earn 100
Convert the following:
Chloroethane to methanamine.
Important Questions on Amines
HARD
Starting from propanoic acid, the following reactions were carried out
Propanoic acid
What is the compound ?
EASY
EASY
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
HARD
In the reaction,
end product is
MEDIUM
MEDIUM
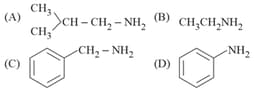
EASY
EASY
EASY
MEDIUM

