EASY
Earn 100
Cooking is fast in a pressure cooker, because
(a)Food particles are effectively smashed
(b)Water boils at higher temperature inside the pressure cooker
(c)Food is cooked at constant volume
(d)Loss of heat due to radiation is minimum
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
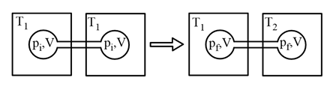
EASY
MEDIUM
MEDIUM
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
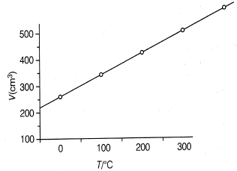
The volume of the gas at is larger than that at by a factor of
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
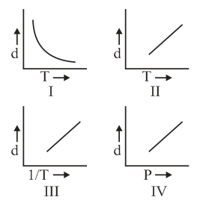
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
MEDIUM
HARD
MEDIUM
[Gas constant, ]
MEDIUM
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

