MEDIUM
Earn 100
Correct order of bond angle is :-
(a)
(b)
(c)
(d)
(a)
(b)
(c)Only
(d)Only
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
Consider the molecules and . Which of the given statements is false?
MEDIUM
The type of hybridization and no. of lone pair(s) of electron of in , respectively, are:
MEDIUM
The species in which the N atom is a state of sp hybridization is:
MEDIUM
Which one the following species has plane triangular shape?
MEDIUM
The group having triangular planar structure is:
HARD
The ion that has hybridization for the central atom is:
EASY
The order of electronegativity of carbon in and hybridized states follows
EASY
The hybridisation of phosphorous in is
MEDIUM
hybridization is not displayed by
EASY
Which of the following pairs of compounds is isoelectronic and isostructural?
EASY
The number of hybrid orbitals in a molecule of benzene is:
EASY
Which of the following conversions involves change in both shape and hybridisation?
EASY
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
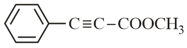
EASY
The bond angle H-X-H is the greatest in the compound :
MEDIUM
Among the compounds shown below which one revealed a linear structure?
EASY
The percentage of s-character in the hybrid orbitals of nitrogen in and respectively are:
EASY
In molecule, the hybridizations of carbon and oxygen atoms are respectively
EASY
The species having bond angles of is
EASY
The compounds containing sp hybridized carbon atom are
(i)
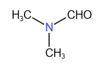 (ii)
(ii) 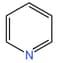
(iii) (iv)
MEDIUM
The hybridisation of atomic orbitals of nitrogen in and , respectively are:

