HARD
11th CBSE
IMPORTANT
Earn 100
Correct pair of compounds which give blue colouration/ precipitate and white precipitate, respectively, when their Lassaigne's test is separately done is
(a)
(b)
(c)
(d)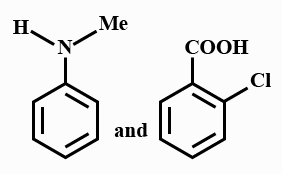

26.09% studentsanswered this correctly

Important Questions on Organic Chemistry - Some Basic Principles and Techniques
HARD
11th CBSE
IMPORTANT
of an organic compound containing and on combustion yields of and of . If one mole of a compound weighs , then molecular formula of the compound is
HARD
11th CBSE
IMPORTANT
An organic compound having molecular mass is found to contain , and , while rest is oxygen. On heating, it gives ammonia along with a solid residue. The solid residue gave violet colour with alkaline copper sulphate solution. The compound is
HARD
11th CBSE
IMPORTANT
In Duma's method for estimation of nitrogen, of an organic compound gave of nitrogen collected at temperature and pressure. If the aqueous tension at is , the percentage of nitrogen in the compound is
HARD
11th CBSE
IMPORTANT
Which of the following compounds will be suitable for Kjeldahl's method for nitrogen estimation?
HARD
11th CBSE
IMPORTANT
In Kjeldahl's method for the estimation of nitrogen present in soil sample, ammonia evolved from of sample neutralized of . The percentage of nitrogen in the soil is
HARD
11th CBSE
IMPORTANT
The ammonia evolved from the treatment of of an organic compound for the estimation of nitrogen was passed in of sulphuric acid. The excess of acid required of sodium hydroxide solution for complete neutralization. The organic compound is
HARD
11th CBSE
IMPORTANT
of an organic compound containing nitrogen was digested according to Kjeldahl’s method and the evolved ammonia was absorbed in of solution. The excess of the acid required of solution for complete neutralization, The percentage of nitrogen in the compound is
HARD
11th CBSE
IMPORTANT
A sample of of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in of The remaining acid after neutralisation by ammonia consumed of . The percentage of nitrogen in the organic compound is
