MEDIUM
Earn 100
Critical temperature and inversion temperature of nitrogen are and , respectively. If Nitrogen gas is allowed to expand adiabatically at its temperature:
(a)increases
(b)decreases
(c)remains same
(d)can't predict
50% studentsanswered this correctly
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
Observe the following properties:
Volume, enthalpy, density, temperature, heat capacity, pressure, internal energy.
The number of extensive properties in the above list is
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
Among the following the number of state variable is
Internal energy
Volume
Heat
Enthalpy
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
The total number of intensive properties from the following is...........
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
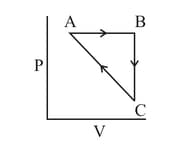
Heat absorbed by the system during process is
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
i)
ii)
iii)
iv)

