EASY
AS and A Level
IMPORTANT
Earn 100
Crystals of sodium chloride have a lattice structure.
Explain the following property of sodium chloride:
Sodium chloride conducts electricity when molten but not when solid.

Important Questions on States of Matter
EASY
AS and A Level
IMPORTANT
Crystals of sodium chloride have a lattice structure.
Explain the following property of sodium chloride:
Sodium chloride is hard but brittle.
EASY
AS and A Level
IMPORTANT
The diagram shows some allotropes of carbon.
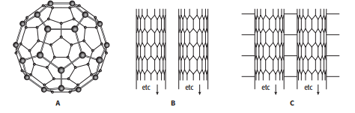
Explain in terms of structure and bonding why structure A is gaseous at 800 °C, but diamond is not.
EASY
AS and A Level
IMPORTANT
The diagram shows some allotropes of carbon.
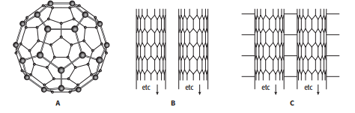
Structure B shows an allotrope of carbon in the form of tubes.
Describe the similarities and differences between structure B and graphite.
EASY
AS and A Level
IMPORTANT
The diagram shows some allotropes of carbon.
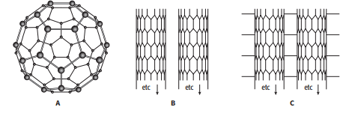
Structure C is stronger than structure B when a force is applied in the same direction as the long axis of the tube.
Explain why structure C is stronger.
